Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24)
- PMID: 17445775
- PMCID: PMC3371634
- DOI: 10.1016/j.bcp.2007.03.002
Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24)
Abstract
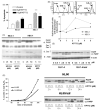
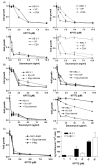
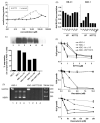
Recently, we have introduced [tris(1,10-phenanthroline)lanthanum(III)] trithiocyanate (KP772, FFC24) as a new lanthanum compound which has promising anticancer properties in vivo and in vitro. Aim of this study was to investigate the impact of ABC transporter-mediated multidrug resistance (MDR) on the anticancer activity of KP772. Here, we demonstrate that all MDR cell models investigated, overexpressing ABCB1 (P-glycoprotein), ABCC1 (multidrug resistance protein 1), or ABCG2 (breast cancer resistance protein) either due to drug selection or gene transfection, were significantly hypersensitive against KP772. Using ABCB1-overexpressing KBC-1 cells as MDR model, KP772 hypersensitivity was demonstrated to be based on stronger apoptosis induction and/or cell cycle arrest at unaltered cellular drug accumulation. KP772 did neither stimulate ABCB1 ATPase activity nor alter rhodamine 123 accumulation arguing against a direct interaction with ABCB1. Accordingly, several drug resistance modulators did not sensitize but rather protect MDR cells against KP772-induced cytotoxicity. Moreover, long-term KP772 treatment of KBC-1 cells at subtoxic concentrations led within 20 passages to a complete loss of drug resistance based on blocked MDR1 gene expression. When exposing parental KB-3-1 cells to subtoxic, stepwise increasing KP772 concentrations, we observed, in contrast to several other metallo-drugs, no acquisition of KP772 resistance. Summarizing, our data demonstrate that KP772 is hyperactive in MDR cells and might have chemosensitizing properties by blocking ABCB1 expression. Together with the disability of tumor cells to acquire KP772 resistance, our data suggest that KP772 should be especially active against notoriously drug-resistant tumor types and as second line treatment after standard chemotherapy failure.
Figures



References
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. - PubMed
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. - PubMed
-
- Heffeter P, Jakupec MA, Korner W, Wild S, von Keyserlingk NG, Elbling L, et al. Anticancer activity of the lanthanum compound [tris(1,10-phenanthroline)lanthanum(III)]trithiocyanate (KP772; FFC24) Biochem Pharmacol. 2006;71:426–40. - PubMed
-
- Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, et al. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986;261:7762–70. - PubMed
-
- McGrath T, Center MS. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988;48:3959–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

