Ciliary neurotrophic factor is not required for terminal sprouting and compensatory reinnervation of neuromuscular synapses: re-evaluation of CNTF null mice
- PMID: 17445802
- PMCID: PMC1931609
- DOI: 10.1016/j.expneurol.2007.03.011
Ciliary neurotrophic factor is not required for terminal sprouting and compensatory reinnervation of neuromuscular synapses: re-evaluation of CNTF null mice
Abstract
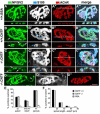
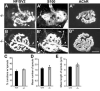
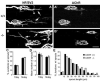
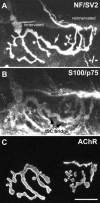
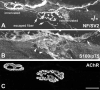
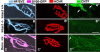
Loss of synaptic activity or innervation induces sprouting of intact motor nerve terminals that adds or restores nerve-muscle connectivity. Ciliary neurotrophic factor (CNTF) and terminal Schwann cells (tSCs) have been implicated as molecular and cellular mediators of the compensatory process. We wondered if the previously reported lack of terminal sprouting in CNTF null mice was due to abnormal reactivity of tSCs. To this end, we examined nerve terminal and tSC responses in CNTF null mice using experimental systems that elicited extensive sprouting in wildtype mice. Contrary to the previous report, we found that motor nerve terminals in the null mice sprout extensively in response to major sprouting-stimuli such as exogenously applied CNTF per se, botulinum toxin-elicited paralysis, and partial denervation by L4 spinal root transection. In addition, the number, length and growth patterns of terminal sprouts, and the extent of reinnervation by terminal or nodal sprouts, were similar in wildtype and null mice. tSCs in the null mice were also reactive to the sprouting-stimuli, elaborating cellular processes that accompanied terminal sprouts or guided reinnervation of denervated muscle fibers. Lastly, CNTF was absent in quiescent tSCs in intact, wildtype muscles and little if any was detected in reactive tSCs in denervated muscles. Thus, CNTF is not required for induction of nerve terminal sprouting, for reactivation of tSCs, and for compensatory reinnervation after nerve injury. We interpret these results to support the notion that compensatory sprouting in adult muscles is induced primarily by contact-mediated mechanisms, rather than by diffusible factors.
Figures
References
-
- Albani M, Lowrie MB, Vrbova G. Reorganization of motor units in reinnervated muscles of the rat. J. Neurol. Sci. 1988;88:195–206. - PubMed
-
- Angaut-Petit D, Molgo J, Comella JX, Faille L, Tabti N. Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience. 1990;37:799–808. - PubMed
-
- Angaut-Petit D, Molgo J, Connold AL, Faille L. The levator auris longus muscle of the mouse: a convenient preparation for studies of short- and long-term presynaptic effects of drugs or toxins. Neurosci. Lett. 1987;82:83–88. - PubMed
-
- Angaut-Petit D, Molgo J, Faille L, Juzans P, Takahashi M. Incorporation of synaptotagmin II to the axolemma of botulinum type-A poisoned mouse motor endings during enhanced quantal acetylcholine release. Brain Res. 1998;797:357–360. - PubMed
-
- Booth CM, Kemplay SK, Brown MC. An antibody to neural cell adhesion molecule impairs motor nerve terminal sprouting in a mouse muscle locally paralysed with botulinum toxin. Neuroscience. 1990;35:85–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

