Structure of a high-affinity "mimotope" peptide bound to HIV-1-neutralizing antibody b12 explains its inability to elicit gp120 cross-reactive antibodies
- PMID: 17445828
- PMCID: PMC1995417
- DOI: 10.1016/j.jmb.2007.01.060
Structure of a high-affinity "mimotope" peptide bound to HIV-1-neutralizing antibody b12 explains its inability to elicit gp120 cross-reactive antibodies
Abstract
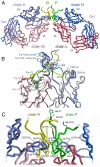
The human antibody b12 recognizes a discontinuous epitope on gp120 and is one of the rare monoclonal antibodies that neutralize a broad range of primary human immunodeficiency virus type 1 (HIV-1) isolates. We previously reported the isolation of B2.1, a dimeric peptide that binds with high specificity to b12 and competes with gp120 for b12 antibody binding. Here, we show that the affinity of B2.1 was improved 60-fold over its synthetic-peptide counterpart by fusing it to the N terminus of a soluble protein. This affinity, which is within an order of magnitude of that of gp120, probably more closely reflects the affinity of the phage-borne peptide. The crystal structure of a complex between Fab of b12 and B2.1 was determined at 1.8 A resolution. The structural data allowed the differentiation of residues that form critical contacts with b12 from those required for maintenance of the antigenic structure of the peptide, and revealed that three contiguous residues mediate B2.1's critical contacts with b12. This single region of critical contact between the B2.1 peptide and the b12 paratope is unlikely to mimic the discontinuous key binding residues involved in the full b12 epitope for gp120, as previously identified by alanine scanning substitutions on the gp120 surface. These structural observations are supported by experiments that demonstrate that B2.1 is an ineffective immunogenic mimic of the b12 epitope on gp120. Indeed, an extensive series of immunizations with B2.1 in various forms failed to produce gp120 cross-reactive sera. The functional and structural data presented here, however, suggest that the mechanism by which b12 recognizes the two antigens is very different. Here, we present the first crystal structure of peptide bound to an antibody that was originally raised against a discontinuous protein epitope. Our results highlight the challenge of producing immunogens that mimic discontinuous protein epitopes, and the necessity of combining complementary experimental approaches in analyzing the antigenic and immunogenic properties of putative molecular mimics.
Figures


References
-
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, Lamacchia M, Garratty E, Richard ES, Bryson YJ, Cao Y, Moore JP, Ho DD, Barbas CF., 3rd Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. - PubMed
-
- Kessler JA, 2nd, McKenna PM, Emini EA, Chan CP, Patel MD, Gupta SK, Mark GE, Barbas CF, 3rd, Burton DR, Conley AJ. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res. Hum. Retroviruses. 1997;13:575–582. - PubMed
-
- Trkola A, Pomales AB, Yuan H, Korber B, Maddon PJ, Allaway GP, Katinger H, Barbas CF, 3rd, Burton DR, Ho DD, Moore JP. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 1995;69:6609–6617. - PMC - PubMed
-
- Parren PWHI, Ditzel HJ, Gulizia RJ, Binley JM, Barbas CF, 3rd, Burton DR, Mosier DE. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–6. - PubMed
-
- Gauduin MC, Parren PW, Weir R, Barbas CF, 3rd, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 1997;3:1389–1393. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

