Control of basal extracellular adenosine concentration in rat cerebellum
- PMID: 17446223
- PMCID: PMC2075308
- DOI: 10.1113/jphysiol.2007.132050
Control of basal extracellular adenosine concentration in rat cerebellum
Abstract
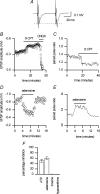
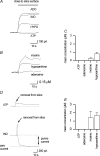
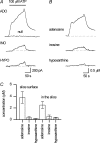
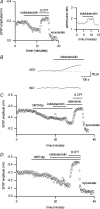
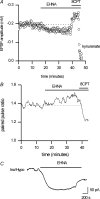
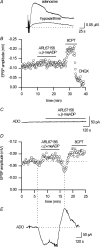
To re-examine how the basal extracellular concentration of adenosine is regulated in acutely isolated cerebellar slices we have combined electrophysiological and microelectrode biosensor measurements. In almost all cases, synaptic transmission was tonically inhibited by adenosine acting via A1 receptors. By contrast, in most slices, the biosensors did not measure an adenosine tone but did record a spatially non-uniform extracellular tone of the downstream metabolites (inosine and hypoxanthine). Most of the extracellular hypoxanthine arose from the metabolism of inosine by ecto-purine nucleoside phosphorylase (PNP). Adenosine kinase was the major determinant of adenosine levels, as its inhibition increased both adenosine concentration and A1 receptor-mediated synaptic inhibition. Breakdown of adenosine by adenosine deaminase was the major source of the inosine/hypoxanthine tone. However adenosine deaminase played a minor role in determining the level of adenosine at synapses, suggesting a distal location. Blockade of adenosine transport (by NBTI/dipyridamole) had inconsistent effects on basal levels of adenosine and synaptic transmission. Unexpectedly, application of NBTI/dipyridamole prevented the efflux of adenosine resulting from block of adenosine kinase at only a subset of synapses. We conclude that there is spatial variation in the functional expression of NBTI/dipyridamole-sensitive transporters. The increased spatial and temporal resolution of the purine biosensor measurements has revealed the complexity of the control of adenosine and purine tone in the cerebellum.
Figures


References
-
- Agarwal RP. Inhibitors of adenosine deaminase. Pharmacol Ther. 1982;17:399–429. - PubMed
-
- Anderson CM, Baldwin SA, Young JD, Cass CE, Parkinson FE. Distribution of mRNA encoding a nitrobenzylthioinosine-insensitive nucleoside transporter (ENT2) in rat brain. Brain Res Mol Brain Res. 1999a;70:293–297. - PubMed
-
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, Parkinson FE. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. J Neurochem. 1999b;73:867–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

