Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans
- PMID: 17446226
- PMCID: PMC2075265
- DOI: 10.1113/jphysiol.2007.128447
Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans
Abstract
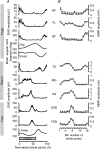
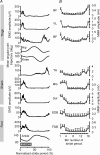
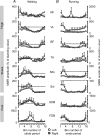
Motor responses evoked by stimulating the spinal cord percutaneously between the T11 and T12 spinous processes were studied in eight human subjects during walking and running. Stimulation elicited responses bilaterally in the biceps femoris, vastus lateralis, rectus femoris, medial gastrocnemius, soleus, tibialis anterior, extensor digitorum brevis and flexor digitorum brevis. The evoked responses were consistent with activation of Ia afferent fibres through monosynaptic neural circuits since they were inhibited when a prior stimulus was given and during tendon vibration. Furthermore, the soleus motor responses were inhibited during the swing phase of walking as observed for the soleus H-reflex elicited by tibial nerve stimulation. Due to the anatomical site and the fibre composition of the peripheral nerves it is difficult to elicit H-reflex in leg muscles other than the soleus, especially during movement. In turn, the multisegmental monosynaptic responses (MMR) technique provides the opportunity to study modulation of monosynaptic reflexes for multiple muscles simultaneously. Phase-dependent modulation of the MMR amplitude throughout the duration of the gait cycle period was observed in all muscles studied. The MMR amplitude was large when the muscle was activated whereas it was generally reduced, or even suppressed, when the muscle was quiescent. However, during running, there was a systematic anticipatory increase in the amplitude of the MMR at the end of swing in all proximal and distal extensor muscles. The present findings therefore suggest that there is a general control scheme by which the transmission in the monosynaptic neural circuits is modulated in all leg muscles during stepping so as to meet the requirement of the motor task.
Figures



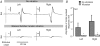



References
-
- Agduhr E. Vergleich der Neuritenanzahl in den Wurzeln der Spinalnerven bei Kröte, Maus, Hund und Mensch. Z Anat. 1934;102:194–210.
-
- Andersen JB, Sinkjaer T. The stretch reflex and H-reflex of the human soleus muscle during walking. Motor Control. 1999;3:151–157. - PubMed
-
- Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127:2232–2246. - PubMed
-
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984;52:435–448. - PubMed
-
- Capaday C. The special nature of human walking and its neural control. Trends Neurosci. 2002;25:370–376. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

