Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses
- PMID: 17447841
- PMCID: PMC1853117
- DOI: 10.1371/journal.ppat.0030057
Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses
Abstract
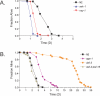
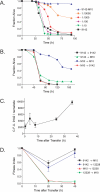
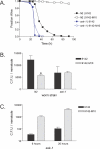
Staphylococcus epidermidis and Staphylococcus aureus are leading causes of hospital-acquired infections that have become increasingly difficult to treat due to the prevalence of antibiotic resistance in these organisms. The ability of staphylococci to produce biofilm is an important virulence mechanism that allows bacteria both to adhere to living and artificial surfaces and to resist host immune factors and antibiotics. Here, we show that the icaADBC locus, which synthesizes the biofilm-associated polysaccharide intercellular adhesin (PIA) in staphylococci, is required for the formation of a lethal S. epidermidis infection in the intestine of the model nematode Caenorhabditis elegans. Susceptibility to S. epidermidis infection is influenced by mutation of the C. elegans PMK-1 p38 mitogen-activated protein (MAP) kinase or DAF-2 insulin-signaling pathways. Loss of PIA production abrogates nematocidal activity and leads to reduced bacterial accumulation in the C. elegans intestine, while overexpression of the icaADBC locus in S. aureus augments virulence towards nematodes. PIA-producing S. epidermidis has a significant survival advantage over ica-deficient S. epidermidis within the intestinal tract of wild-type C. elegans, but not in immunocompromised nematodes harboring a loss-of-function mutation in the p38 MAP kinase pathway gene sek-1. Moreover, sek-1 and pmk-1 mutants are equally sensitive to wild-type and icaADBC-deficient S. epidermidis. These results suggest that biofilm exopolysaccharide enhances virulence by playing an immunoprotective role during colonization of the C. elegans intestine. These studies demonstrate that C. elegans can serve as a simple animal model for studying host-pathogen interactions involving staphylococcal biofilm exopolysaccharide and suggest that the protective activity of biofilm matrix represents an ancient conserved function for resisting predation.
Conflict of interest statement
Figures





References
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. - PubMed
-
- Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. - PubMed
-
- Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, et al. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: Genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. - PMC - PubMed
-
- Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–1378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

