Temporal onset of hypoxia and oxidative stress after pulmonary irradiation
- PMID: 17448873
- PMCID: PMC1939695
- DOI: 10.1016/j.ijrobp.2006.12.056
Temporal onset of hypoxia and oxidative stress after pulmonary irradiation
Abstract
Purpose: To investigate the temporal onset of hypoxia following irradiation, and to show how it relates to pulmonary vascular damage, macrophage accumulation, and the production of reactive oxygen species and cytokines. Our previous studies showed that tissue hypoxia in the lung after irradiation contributed to radiation-induced injury.
Methods and materials: Female Fisher 344 rats were irradiated to the right hemithorax with a single dose of 28 Gy. Serial studies were performed up to 20 weeks following irradiation. Radionuclide lung-perfusion studies were performed to detect changes in pulmonary vasculature. Immunohistochemical studies were conducted to study macrophages, tissue hypoxia (carbonic anhydrase-9 marker), oxidative stress (8-hydroxy-2'-deoxyguanosine), and the expression of profibrogenic (transforming growth factor-beta [TGF-beta]) and proangiogenic (vascular endothelial growth factor [VEGF]) cytokines.
Results: Significant changes in lung perfusion along with tissue hypoxia were observed 3 days after irradiation. Significant oxidative stress was detected 1 week after radiation, whereas macrophages started to accumulate at 4 weeks. A significant increase in TGF-beta expression was seen within 1 day after radiation, and for VEGF at 2 weeks after radiation. Levels of hypoxia, oxidative stress, and both cytokines continued to rise with time after irradiation. The steepest increase correlated with vast macrophage accumulation.
Conclusions: Early changes in lung perfusion, among other factors initiate, the development of hypoxia and chronic oxidative stress after irradiation. Tissue hypoxia is associated with a significant increase in the activation of macrophages and their continuous production of reactive oxygen species, stimulating the production of fibrogenic and angiogenic cytokines, and maintaining the development of chronic radiation-induced lung injury.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflict of interest.
Figures
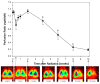

activated macrophages (ED-1)
hypoxia (CA-9)
ROS (8-OHdG)
VEGF
TGF-β
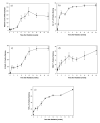
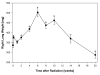
Number of activated macrophages (ED-1): A significant increase in macrophages was seen 4 weeks after irradiation (p=0.0231) and peaked at 10 weeks (p=0.00979).
Hypoxia (CA-9): A significant increase in CA-9 expression was already seen 3 days after irradiation (p=0.000327) and increased further with time.
ROS (8-OHdG): A significant increase in 8-OHdG expression started 1 week after irradiation (p=0.0097) and increased further with time.
VEGF: A significant increase in VEGF expression started 2 weeks after irradiation (p=0.0284).
TGF-β: A significant increase in TGF-β expression was already seen 1 day after irradiation (p=0.00146) and continuously increased further with time.

References
-
- Abratt RP, Morgan GW. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer. 2002;35:103–109. - PubMed
-
- Roach M, 3rd, Gandara DR, Yuo HS, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13:2606–2612. - PubMed
-
- Moosavi H, McDonald S, Rubin P, et al. Early radiation dose-response in lung: an ultrastructural study. Int J Radiat Oncol Biol Phys. 1977;2:921–931. - PubMed
-
- Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 1980;6:345–347. - PubMed
-
- Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophage and the septal fibroblast. Int J Radiat Oncol Biol Phys. 1992;24:93–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

