pH-dependent association of enolase and glyceraldehyde-3-phosphate dehydrogenase of Lactobacillus crispatus with the cell wall and lipoteichoic acids
- PMID: 17449624
- PMCID: PMC1913374
- DOI: 10.1128/JB.00378-07
pH-dependent association of enolase and glyceraldehyde-3-phosphate dehydrogenase of Lactobacillus crispatus with the cell wall and lipoteichoic acids
Erratum in
- J Bacteriol. 2007 Aug;189(15):5788. Kupannen, Veera [corrected to Kuparinen, Veera]
Abstract
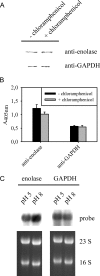
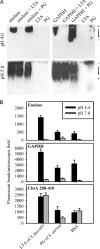
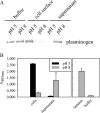
The plasminogen-binding proteins enolase and glyceraldehyde-3-phosphate dehydrogenase of Lactobacillus crispatus were localized on the cell surface at pH 5 but released into the medium at an alkaline pH. These proteins bound to lipoteichoic acids at a pH below their isoelectric point. The results indicate that lactobacilli rapidly modify their surface properties in response to changes in pH.
Figures


References
-
- Alvarez, R. A., M. W. Blaylock, and J. B. Baseman. 2003. Surface localized glyceraldehyde-3-phosphate dehydrogenase of Mycoplasma genitalium binds mucin. Mol. Microbiol. 48:1417-1425. - PubMed
-
- Antikainen, J., L. Anton, J. Sillanpää, and T. K. Korhonen. 2002. Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol. Microbiol. 46:381-394. - PubMed
-
- Bergmann, S., M. Rohde, K. T. Preissner, and S. Hammerschmidt. 2005. The nine residue plasminogen-binding motif of the pneumococcal enolase is the major cofactor of plasmin-mediated degradation of extracellular matrix, dissolution of fibrin and transmigration. Thromb. Haemostasis 94:304-311. - PubMed
-
- Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273-1287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

