The stoichiometry of P2X2/6 receptor heteromers depends on relative subunit expression levels
- PMID: 17449665
- PMCID: PMC1896263
- DOI: 10.1529/biophysj.106.101048
The stoichiometry of P2X2/6 receptor heteromers depends on relative subunit expression levels
Abstract
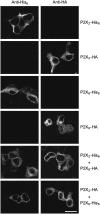
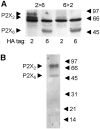
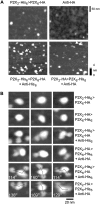
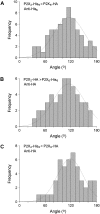
Fast synaptic transmission involves the operation of ionotropic receptors, which are often composed of at least two types of subunit. We have developed a method, based on atomic force microscopy imaging to determine the stoichiometry and subunit arrangement within ionotropic receptors. We showed recently that the P2X(2) receptor for ATP is expressed as a trimer but that the P2X(6) subunit is unable to oligomerize. In this study we addressed the subunit stoichiometry of heteromers containing both P2X(2) and P2X(6) subunits. We transfected tsA 201 cells with both P2X(2) and P2X(6) subunits, bearing different epitope tags. We manipulated the transfection conditions so that either P2X(2) or P2X(6) was the predominant subunit expressed. By atomic force microscopy imaging of isolated receptors decorated with antiepitope antibodies, we demonstrate that when expression of the P2X(2) subunit predominates, the receptors contain primarily 2 x P2X(2) subunits and 1 x P2X(6) subunit. In contrast, when the P2X(6) subunit predominates, the subunit stoichiometry of the receptors is reversed. Our results show that the composition of P2X receptor heteromers is plastic and dependent on the relative subunit expression levels. We suggest that this property of receptor assembly might introduce an additional layer of subtlety into P2X receptor signaling.
Figures

References
-
- Lester, H. A., M. I. Dibas, D. S. Dahan, J. F. Leite, and D. A. Dougherty. 2004. Cys-loop receptors: new twists and turns. Trends Neurosci. 27:329–336. - PubMed
-
- Furukawa, H., S. K. Singh, R. Mancusso, and E. Gouaux. 2005. Subunit arrangement and function in NMDA receptors. Nature. 438:185–192. - PubMed
-
- North, R. A. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82:1013–1067. - PubMed
-
- Zwart, R., and H. P. M. Vijverberg. 1998. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol. Pharmacol. 54:1124–1131. - PubMed
-
- Nelson, M. E., A. Kuryatov, C. H. Choi, Y. Zhou, and J. Lindstrom. 2003. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 63:332–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

