Conformation of the EPEC Tir protein in solution: investigating the impact of serine phosphorylation at positions 434/463
- PMID: 17449672
- PMCID: PMC1896257
- DOI: 10.1529/biophysj.106.101766
Conformation of the EPEC Tir protein in solution: investigating the impact of serine phosphorylation at positions 434/463
Abstract
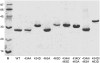
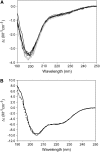
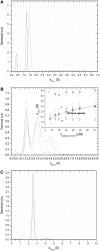
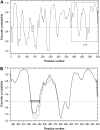
The translocated intimin receptor (Tir) is a key virulence factor of enteropathogenic Escherichia coli and related bacteria. During infection, Tir is translocated via a type III secretion system into host intestinal epithelial cells, where it inserts into the target cell membrane and acts as a receptor for the bacterial adhesin intimin. The effects of phosphorylation by cAMP-dependent kinase at two serine residues (Ser-434 and Ser-463) within the C-terminal domain of Tir, which may be involved in mediating structural/electrostatic changes in the protein to promote membrane insertion or intermolecular interactions, have previously been investigated. This study has focused on defining the conformation of Tir in solution and assessing any conformational changes associated with serine phosphorylation at positions 434/463. In addition to phosphorylated protein, combinations of Ala (unphosphorylatable) and Asp (phosphate-mimic) mutations of Ser-434 and Ser-463 have been generated, and a range of techniques (sodium dodecyl sulfate polyacrylamide gel electrophoresis, circular dichroism spectroscopy, analytical ultracentrifugation) used to further dissect the structural role and functional implications of changes in residue size/charge at these positions. The results have shown that under physiological NaCl concentrations, Tir is a monomer and adopts a highly elongated state in solution, consistent with a natively unfolded conformation. Despite this, perturbations in the structure in response to buffer conditions and the nature of the residues at positions 434 and 463 are apparent, and may be functionally relevant.
Figures

Similar articles
-
Biophysical analysis of the EPEC translocated intimin receptor-binding domain.Biochem Biophys Res Commun. 2007 Nov 3;362(4):1073-8. doi: 10.1016/j.bbrc.2007.08.123. Epub 2007 Aug 30. Biochem Biophys Res Commun. 2007. PMID: 17825257
-
Dual infection system identifies a crucial role for PKA-mediated serine phosphorylation of the EPEC-Tir-injected effector protein in regulating Rac1 function.Cell Microbiol. 2009 Aug;11(8):1254-71. doi: 10.1111/j.1462-5822.2009.01330.x. Epub 2009 Apr 30. Cell Microbiol. 2009. PMID: 19438518
-
Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli.Cell Microbiol. 1999 Jul;1(1):7-17. doi: 10.1046/j.1462-5822.1999.00001.x. Cell Microbiol. 1999. PMID: 11207537
-
The N-terminus of enteropathogenic Escherichia coli (EPEC) Tir mediates transport across bacterial and eukaryotic cell membranes.Mol Microbiol. 2002 Nov;46(3):855-68. doi: 10.1046/j.1365-2958.2002.03214.x. Mol Microbiol. 2002. PMID: 12410841
-
Phosphoserine modification of the enteropathogenic Escherichia coli Tir molecule is required to trigger conformational changes in Tir and efficient pedestal elongation.Mol Microbiol. 2001 Dec;42(5):1269-80. doi: 10.1046/j.1365-2958.2001.02693.x. Mol Microbiol. 2001. PMID: 11886558
Cited by
-
Contribution of Crk adaptor proteins to host cell and bacteria interactions.Biomed Res Int. 2014;2014:372901. doi: 10.1155/2014/372901. Epub 2014 Nov 25. Biomed Res Int. 2014. PMID: 25506591 Free PMC article. Review.
-
Distinct phosphorylation requirements regulate cortactin activation by TirEPEC and its binding to N-WASP.Cell Commun Signal. 2009 May 6;7:11. doi: 10.1186/1478-811X-7-11. Cell Commun Signal. 2009. PMID: 19419567 Free PMC article.
-
On computational approaches for size-and-shape distributions from sedimentation velocity analytical ultracentrifugation.Eur Biophys J. 2010 Jul;39(8):1261-75. doi: 10.1007/s00249-009-0545-7. Epub 2009 Oct 6. Eur Biophys J. 2010. PMID: 19806353 Free PMC article.
-
Sequence Polymorphism and Intrinsic Structural Disorder as Related to Pathobiological Performance of the Helicobacter pylori CagA Oncoprotein.Toxins (Basel). 2017 Apr 13;9(4):136. doi: 10.3390/toxins9040136. Toxins (Basel). 2017. PMID: 28406453 Free PMC article. Review.
-
BrisSynBio: a BBSRC/EPSRC-funded Synthetic Biology Research Centre.Biochem Soc Trans. 2016 Jun 15;44(3):689-91. doi: 10.1042/BST20160004. Biochem Soc Trans. 2016. PMID: 27284028 Free PMC article.
References
-
- Kenny, B. 2002. Enteropathogenic Escherichia coli (EPEC)–a crafty subversive little bug. Microbiology. 148:1967–1978. - PubMed
-
- Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 91:511–520. - PubMed
-
- Kenny, B. 1999. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31:1229–1241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

