Diversity and function of Chloroflexus-like bacteria in a hypersaline microbial mat: phylogenetic characterization and impact on aerobic respiration
- PMID: 17449697
- PMCID: PMC1932729
- DOI: 10.1128/AEM.02532-06
Diversity and function of Chloroflexus-like bacteria in a hypersaline microbial mat: phylogenetic characterization and impact on aerobic respiration
Abstract
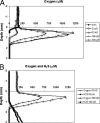
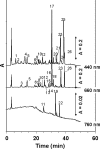
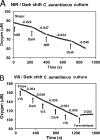
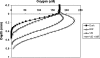
We studied the diversity of Chloroflexus-like bacteria (CLB) in a hypersaline phototrophic microbial mat and assayed their near-infrared (NIR) light-dependent oxygen respiration rates. PCR with primers that were reported to specifically target the 16S rRNA gene from members of the phylum Chloroflexi resulted in the recovery of 49 sequences and 16 phylotypes (sequences of the same phylotype share more than 96% similarity), and 10 of the sequences (four phylotypes) appeared to be related to filamentous anoxygenic phototrophic members of the family Chloroflexaceae. Photopigment analysis revealed the presence of bacteriochlorophyll c (BChlc), BChld, and gamma-carotene, pigments known to be produced by phototrophic CLB. Oxygen microsensor measurements for intact mats revealed a NIR (710 to 770 nm) light-dependent decrease in aerobic respiration, a phenomenon that we also observed in an axenic culture of Chloroflexus aurantiacus. The metabolic ability of phototrophic CLB to switch from anoxygenic photosynthesis under NIR illumination to aerobic respiration under non-NIR illumination was further used to estimate the contribution of these organisms to mat community respiration. Steady-state oxygen profiles under dark conditions and in the presence of visible (VIS) light (400 to 700 nm), NIR light (710 to 770 nm), and VIS light plus NIR light were compared. NIR light illumination led to a substantial increase in the oxygen concentration in the mat. The observed impact on oxygen dynamics shows that CLB play a significant role in the cycling of carbon in this hypersaline microbial mat ecosystem. This study further demonstrates that the method applied, a combination of microsensor techniques and VIS and NIR illumination, allows rapid establishment of the presence and significance of CLB in environmental samples.
Figures

References
-
- Abed, R. M. M., and F. Garcia-Pichel. 2001. Long-term compositional changes after transplant in a microbial mat cyanobacterial community revealed using a polyphasic approach. Environ. Microbiol. 3:53-62. - PubMed
-
- Bjornsson, L., P. Hugenholtz, G. W. Tyson, and L. L. Blackall. 2002. Filamentous Chloroflexi (green non-sulfur bacteria) are abundant in wastewater treatment processes with biological nutrient removal. Microbiology 149:2309-2318. - PubMed
-
- Buffan-Dubau, E., O. Pringault, and R. DeWit. 2001. Artificial cold-adapted microbial mats cultured from Antarctic lake samples. 1. Formation and structure. Aquat. Microb. Ecol. 26:115-125.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

