Time sequence of airway remodeling in a mouse model of chronic asthma: the relation with airway hyperresponsiveness
- PMID: 17449921
- PMCID: PMC2693579
- DOI: 10.3346/jkms.2007.22.2.183
Time sequence of airway remodeling in a mouse model of chronic asthma: the relation with airway hyperresponsiveness
Abstract
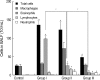
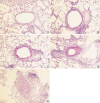
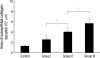
During the course of establishing an animal model of chronic asthma, we tried to elucidate the time sequence of airway hyperresponsiveness (AHR), airway inflammation, airway remodeling, and associated cytokines. Seven-week-old female BALB/c mice were studied as a chronic asthma model using ovalbumin (OVA). After sensitization, mice were exposed twice weekly to aerosolized OVA, and were divided into three groups depending on the duration of 4 weeks, 8 weeks, and 12 weeks. At each time point, airway responsiveness, inflammatory cells, cytokines in bronchoalveolar lavage fluids (BALF), serum OVA-specific IgE, IgG1, IgG2a, and histological examination were carried out. AHR to methacholine, increased levels of OVA-specific IgG1 and IgG2a, and goblet cell hyperplasia were continuously sustained at each time point of weeks. In contrast, we observed a time-dependent decrease in serum OVA-specific IgE, BALF eosinophils, BALF cytokines such as IL-13, transforming growth factor-beta1, and a time-dependent increase in BALF promatrix metalloproteinase-9 and peribronchial fibrosis. In this OVA-induced chronic asthma model, we observed airway remodelings as well as various cytokines and inflammatory cells being involved in different time-dependent manners. However, increased airway fibrosis did not directly correlate with a further increase in airway hyperresponsiveness.
Figures



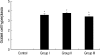

References
-
- Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. - PubMed
-
- Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. - PubMed
-
- Bousquet J, Chanez P, Lacoste JY, White R, Vic P, Godard P, Michel FB. Asthma: a disease remodeling the airways. Allergy. 1992;47:3–11. - PubMed

