Pragmatic trials in primary care. Methodological challenges and solutions demonstrated by the DIAMOND-study
- PMID: 17451599
- PMCID: PMC1865384
- DOI: 10.1186/1471-2288-7-16
Pragmatic trials in primary care. Methodological challenges and solutions demonstrated by the DIAMOND-study
Abstract
Background: Pragmatic randomised controlled trials are often used in primary care to evaluate the effect of a treatment strategy. In these trials it is difficult to achieve both high internal validity and high generalisability. This article will discuss several methodological challenges in designing and conducting a pragmatic primary care based randomised controlled trial, based on our experiences in the DIAMOND-study and will discuss the rationale behind the choices we made. From the successes as well as the problems we experienced the quality of future pragmatic trials may benefit.
Discussion: The first challenge concerned choosing the clinically most relevant interventions to compare and enable blinded comparison, since two interventions had very different appearances. By adding treatment steps to one treatment arm and adding placebo to both treatment arms both internal and external validity were optimized. Nevertheless, although blinding is essential for a high internal validity, it should be warily considered in a pragmatic trial because it decreases external validity. Choosing and recruiting a representative selection of participants was the second challenge. We succeeded in retrieving a representative relatively large patient sample by carefully choosing (few) inclusion and exclusion criteria, by random selection, by paying much attention to participant recruitment and taking the participant's reasons to participate into account. Good and regular contact with the GPs and patients was to our opinion essential. The third challenge was to choose the primary outcome, which needed to reflect effectiveness of the treatment in every day practice. We also designed our protocol to follow every day practice as much as possible, although standardized treatment is usually preferred in trials. The aim of this was our fourth challenge: to limit the number of protocol deviations and increase external validity.
Summary: It is challenging to design and conduct a pragmatic trial. Thanks to thorough preparation, we were able to collect highly valid data. To our opinion, a critical deliberation of where on the pragmatic--explanatory spectrum you want your trial to be on forehand, in combination with consulting publications especially on patient recruitment procedures, has been helpful in conducting a successful trial.
Figures

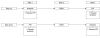

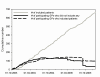
References
-
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, Reed S. Good Research Practices for Cost-Effectiveness Analysis Alongside Clinical Trials: The ISPOR RCT-CEA Task Force Report. Value in Health. 2005;8:521–533. doi: 10.1111/j.1524-4733.2005.00045.x. - DOI - PubMed
-
- Fransen GAJ, R.J.F. L. Registration protocol DIAMOND study (ZONMW 095-03-052) 2006. http://www.clinicaltrials.gov/ct/show/NCT00247715?order=9
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

