Therapy of established B16-F10 melanoma tumors by a single vaccination of CTL/T helper peptides in VacciMax
- PMID: 17451606
- PMCID: PMC1867806
- DOI: 10.1186/1479-5876-5-20
Therapy of established B16-F10 melanoma tumors by a single vaccination of CTL/T helper peptides in VacciMax
Abstract
Background: Melanoma tumors are known to express antigens that usually induce weak immune responses of short duration. Expression of both tumor-associated antigens p53 and TRP2 by melanoma cells raises the possibility of simultaneously targeting more than one antigen in a therapeutic vaccine. In this report, we show that VacciMax (VM), a novel liposome-based vaccine delivery platform, can increase the immunogenicity of melanoma associated antigens, resulting in tumor elimination.
Methods: C57BL/6 mice bearing B16-F10 melanoma tumors were vaccinated subcutaneously 6 days post tumor implantation with a mixture of synthetic peptides (modified p53: 232-240, TRP-2: 181-188 and PADRE) and CpG. Tumor growth was monitored and antigen-specific splenocyte responses were assayed by ELISPOT.
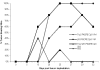
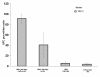
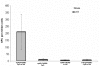
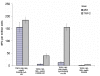
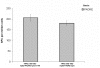
Results: Vaccine formulated in VM increased the number of both TRP2- and p53-specific IFN-gamma producing splenocytes following a single vaccination. Vaccine formulated without VM resulted only in enhanced IFN-gamma producing splenocytes to one CTL epitopes (TRP2:180-188), suggesting that VM overcomes antigen dominance and enhances immunogenicity of multiple epitopes. Vaccination of mice bearing 6-day old B16-F10 tumors with both TRP2 and p53-peptides formulated in VM successfully eradicated tumors in all mice. A control vaccine which contained all ingredients except liposomes resulted in eradication of tumors in no more than 20% of mice.
Conclusion: A single administration of VM is capable of inducing an effective CTL response to multiple tumor-associated antigens. The responses generated were able to reject 6-day old B16-F10 tumors.
Figures
References
-
- Daftarian P, Mansour M, Benoit AC, Pohajdak B, Hoskin DW, Brown RG, Kast WM. Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T helper fusion peptide in a water-in-oil emulsion. Vaccine. 2006;24:5235–5244. doi: 10.1016/j.vaccine.2006.03.079. - DOI - PubMed
-
- Daftarian P, Mansour M, Pohajdak B, Fuentest-Ortega A, Korets-Smith E, MacDonald L, Weir GM, Brown RG, Kast WM. Eradication of large tumors tumors by a single administration of VacciMax-encapsulated CTL-T helper peptides in old mice. J Immunother. 2006;29:646.
-
- Gnjatic S, Cai Z, Viguier M, Chouaib S, Guillet JG, Choppin J. Accumulation of the p53 protein allows recognition by human CTL of a wild-type p53 epitope presented by breast carcinomas and melanomas. J Immunol. 1998;160:328–333. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

