Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli L-asparaginase I
- PMID: 17451745
- PMCID: PMC1991333
- DOI: 10.1016/j.jmb.2007.03.061
Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli L-asparaginase I
Abstract
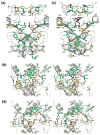
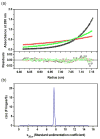
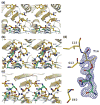
AnsA is the cytoplasmic asparaginase from Escherichia coli involved in intracellular asparagine utilization. Analytical ultracentifugation and X-ray crystallography reveal that AnsA forms a tetrameric structure as a dimer of two intimate dimers. Kinetic analysis of the enzyme reveals that AnsA is positively cooperative, displaying a sigmoidal substrate dependence curve with an [S](0.5) of 1 mM L-asparagine and a Hill coefficient (n(H)) of 2.6. Binding of L-asparagine to an allosteric site was observed in the crystal structure concomitant with a reorganization of the quarternary structure, relative to the apo enzyme. The carboxyl group of the bound asparagine makes salt bridges and hydrogen bonds to Arg240, while the N(delta2) nitrogen interacts with Thr162. Mutation of Arg240 to Ala increases the [S](0.5) value to 5.9 mM, presumably by reducing the affinity of the site for L-asparagine, although the enzyme retains cooperativity. Mutation of Thr162 to Ala results in an active enzyme with no cooperativity. Transmission of the signal from the allosteric site to the active site appears to involve subtle interactions at the dimer-dimer interface and relocation of Gln118 into the vicinity of the active site to position the probable catalytic water molecule. These data define the structural basis for the cooperative regulation of the intracellular asparaginase that is required for proper functioning within the cell.
Figures



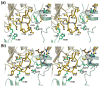
Similar articles
-
Crystal structure of active site mutant of antileukemic L-asparaginase reveals conserved zinc-binding site.FEBS J. 2014 Sep;281(18):4097-111. doi: 10.1111/febs.12906. Epub 2014 Jul 28. FEBS J. 2014. PMID: 25040257
-
A covalently bound catalytic intermediate in Escherichia coli asparaginase: crystal structure of a Thr-89-Val mutant.FEBS Lett. 1996 Jul 22;390(2):211-6. doi: 10.1016/0014-5793(96)00660-6. FEBS Lett. 1996. PMID: 8706862
-
Amino acid residues adjacent to the catalytic cavity of tetramer L-asparaginase II contribute significantly to its catalytic efficiency and thermostability.Enzyme Microb Technol. 2016 Jan;82:15-22. doi: 10.1016/j.enzmictec.2015.08.009. Epub 2015 Aug 18. Enzyme Microb Technol. 2016. PMID: 26672444
-
Why a "benign" mutation kills enzyme activity. Structure-based analysis of the A176V mutant of Saccharomyces cerevisiae L-asparaginase I.Acta Biochim Pol. 1997;44(3):491-504. Acta Biochim Pol. 1997. PMID: 9511960 Review.
-
Phosphoenolpyruvate carboxylase: three-dimensional structure and molecular mechanisms.Arch Biochem Biophys. 2003 Jun 15;414(2):170-9. doi: 10.1016/s0003-9861(03)00170-x. Arch Biochem Biophys. 2003. PMID: 12781768 Review.
Cited by
-
Tackling Critical Catalytic Residues in Helicobacter pylori L-Asparaginase.Biomolecules. 2015 Mar 27;5(2):306-17. doi: 10.3390/biom5020306. Biomolecules. 2015. PMID: 25826146 Free PMC article.
-
Human 60-kDa lysophospholipase contains an N-terminal L-asparaginase domain that is allosterically regulated by L-asparagine.J Biol Chem. 2014 May 9;289(19):12962-75. doi: 10.1074/jbc.M113.545038. Epub 2014 Mar 22. J Biol Chem. 2014. PMID: 24657844 Free PMC article.
-
Identification and structural analysis of an L-asparaginase enzyme from guinea pig with putative tumor cell killing properties.J Biol Chem. 2014 Nov 28;289(48):33175-86. doi: 10.1074/jbc.M114.609552. Epub 2014 Oct 15. J Biol Chem. 2014. PMID: 25320094 Free PMC article.
-
Hyperthermophilic L-Asparaginase from Thermococcus sibiricus and Its Double Mutant with Increased Activity: Insights into Substrate Specificity and Structure.Int J Mol Sci. 2025 Jun 6;26(12):5437. doi: 10.3390/ijms26125437. Int J Mol Sci. 2025. PMID: 40564901 Free PMC article.
-
Recombinant L-Asparaginase from Zymomonas mobilis: A Potential New Antileukemic Agent Produced in Escherichia coli.PLoS One. 2016 Jun 2;11(6):e0156692. doi: 10.1371/journal.pone.0156692. eCollection 2016. PLoS One. 2016. PMID: 27253887 Free PMC article.
References
-
- Cedar H, Schwartz JH. Localization of the two L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967;242:3753–3755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

