Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS
- PMID: 17451814
- PMCID: PMC2709235
- DOI: 10.1016/j.jneuroim.2007.03.002
Antigen specificity of clonally expanded and receptor edited cerebrospinal fluid B cells from patients with relapsing remitting MS
Abstract
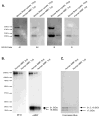
We re-engineered the immunoglobulin rearrangements from clonally expanded CSF B cells of three Multiple Sclerosis patients as Fab fragments, and used three methods to test for their antigen (Ag) specificity. Nine out of ten Fab fragments were reactive to Myelin Basic Protein (MBP). The one Fab that did not react to MBP was a product of receptor editing. Two of the nine MBP reactive Fabs were also reactive to GFAP and CNPase, indicating that these clones were polyreactive. Targeting the mechanisms that allow these self-reactive B cells to reside in the CSF of MS patients may prove to be a potent immunotherapeutic strategy.
Figures



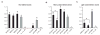

References
-
- Antel J. Multiple sclerosis - emerging concepts of disease pathogenesis. J Neuroimmunol. 1999;98:45–48. - PubMed
-
- Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Annu Neurol. 2000;47:694–706. - PubMed
-
- Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

