Succinate is a paracrine signal for liver damage
- PMID: 17451837
- PMCID: PMC1986575
- DOI: 10.1016/j.jhep.2007.03.016
Succinate is a paracrine signal for liver damage
Abstract
Background/aims: A G-protein-coupled succinate receptor has recently been identified in several tissues, including the liver. The objectives of this work were to determine the hepatic cell types that express this receptor and to determine its physiological role.
Methods: Expression and distribution of the succinate receptor was determined by RT-PCR and confocal immunofluorescence. Biochemical assays were used to measure succinate and cAMP. Cytosolic Ca2+ was monitored in single cells by time-lapse imaging. Western blot was used to study the effect of succinate on activation of hepatic stellate cells.
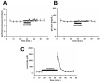
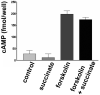
Results: The succinate receptor was expressed in quiescent hepatic stellate cells, and expression decreased with activation. Ischemia induced release of succinate in isolated perfused livers. In contrast to what is observed in cell expression systems, succinate did not inhibit cAMP production or increase cytosolic Ca2+ in primary hepatic stellate cells. However, succinate accelerated stellate cell activation.
Conclusions: Hepatic stellate cells express the succinate receptor. Succinate may behave as a paracrine signal by which ischemic hepatocytes trigger stellate cell activation.
Figures






Similar articles
-
Succinate causes α-SMA production through GPR91 activation in hepatic stellate cells.Biochem Biophys Res Commun. 2015 Aug 7;463(4):853-8. doi: 10.1016/j.bbrc.2015.06.023. Epub 2015 Jun 5. Biochem Biophys Res Commun. 2015. PMID: 26051274
-
Sirtuin 3 (SIRT3) Regulates α-Smooth Muscle Actin (α-SMA) Production through the Succinate Dehydrogenase-G Protein-coupled Receptor 91 (GPR91) Pathway in Hepatic Stellate Cells.J Biol Chem. 2016 May 6;291(19):10277-92. doi: 10.1074/jbc.M115.692244. Epub 2016 Feb 24. J Biol Chem. 2016. PMID: 26912655 Free PMC article.
-
Metformin ameliorates activation of hepatic stellate cells and hepatic fibrosis by succinate and GPR91 inhibition.Biochem Biophys Res Commun. 2018 Jan 22;495(4):2649-2656. doi: 10.1016/j.bbrc.2017.12.143. Epub 2017 Dec 24. Biochem Biophys Res Commun. 2018. PMID: 29278707
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
GPR91: expanding the frontiers of Krebs cycle intermediates.Cell Commun Signal. 2016 Jan 12;14:3. doi: 10.1186/s12964-016-0126-1. Cell Commun Signal. 2016. PMID: 26759054 Free PMC article. Review.
Cited by
-
The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions.Front Endocrinol (Lausanne). 2012 Feb 16;3:22. doi: 10.3389/fendo.2012.00022. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22649411 Free PMC article.
-
Lomatogonium Rotatum for Treatment of Acute Liver Injury in Mice: A Metabolomics Study.Metabolites. 2019 Oct 14;9(10):227. doi: 10.3390/metabo9100227. Metabolites. 2019. PMID: 31615066 Free PMC article.
-
Priming, Triggering, Adaptation and Senescence (PTAS): A Hypothesis for a Common Damage Mechanism of Steatohepatitis.Int J Mol Sci. 2021 Nov 21;22(22):12545. doi: 10.3390/ijms222212545. Int J Mol Sci. 2021. PMID: 34830427 Free PMC article.
-
Mitochondrial Dysfunction in Advanced Liver Disease: Emerging Concepts.Front Mol Biosci. 2021 Nov 23;8:772174. doi: 10.3389/fmolb.2021.772174. eCollection 2021. Front Mol Biosci. 2021. PMID: 34888354 Free PMC article. Review.
-
Succinic Acid Ameliorates Concanavalin A-Induced Hepatitis by Altering the Inflammatory Microenvironment and Expression of BCL-2 Family Proteins.Inflammation. 2024 Dec;47(6):2000-2012. doi: 10.1007/s10753-024-02021-6. Epub 2024 Apr 13. Inflammation. 2024. PMID: 38613638
References
-
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–93. - PubMed
-
- Krebs HA. Rate control of the tricarboxylic acid cycle. Adv Enzyme Regul. 1970;8:335–53. - PubMed
-
- Jhandier MN, Kruglov EA, Lavoie EG, Sevigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem. 2005;280:22986–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

