Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha
- PMID: 17452443
- PMCID: PMC1951471
- DOI: 10.1128/MCB.02279-06
Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha
Abstract
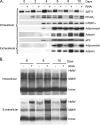
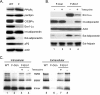
Adiponectin is secreted from adipose tissue in response to metabolic effectors in order to sensitize the liver and muscle to insulin. Reduced circulating levels of adiponectin that usually accompany obesity contribute to the associated insulin resistance. The molecular mechanisms controlling the production of adiponectin are essentially unknown. In this report, we demonstrate that the endoplasmic reticulum (ER) oxidoreductase Ero1-L alpha and effectors modulating peroxisome proliferator-activated receptor gamma (PPAR gamma) and SIRT1 activities regulate secretion of adiponectin from 3T3-L1 adipocytes. Specifically, adiponectin secretion and Ero1-L alpha expression are induced during the early phase of adipogenesis but are then down-regulated during the terminal phase, coincident with an increased expression of SIRT1. Suppression of SIRT1 or activation of PPAR gamma enhances Ero1-L alpha expression and stimulates secretion of high-molecular-weight complexes of adiponectin in mature adipocytes. Suppression of Ero1-L alpha through expression of a corresponding small interfering RNA reduces adiponectin secretion during the differentiation of 3T3-L1 preadipocytes. Moreover, ectopic expression of Ero1-L alpha in Ero1-L alpha-deficient 3T3 fibroblasts stimulates the secretion of adiponectin following their conversion into adipocytes and prevents the suppression of adiponectin secretion in response to activation of SIRT1 by exposure to resveratrol. These findings provide a framework to understand the mechanisms by which adipocytes regulate secretion of adiponectin in response to various metabolic states.
Figures

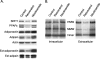





Similar articles
-
Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes.J Biol Chem. 2015 Apr 17;290(16):10143-8. doi: 10.1074/jbc.M115.645416. Epub 2015 Mar 4. J Biol Chem. 2015. PMID: 25739441 Free PMC article.
-
Fisetin up-regulates the expression of adiponectin in 3T3-L1 adipocytes via the activation of silent mating type information regulation 2 homologue 1 (SIRT1)-deacetylase and peroxisome proliferator-activated receptors (PPARs).J Agric Food Chem. 2014 Oct 29;62(43):10468-74. doi: 10.1021/jf502849j. Epub 2014 Oct 15. J Agric Food Chem. 2014. PMID: 25286082
-
Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes.J Gerontol A Biol Sci Med Sci. 2013 Nov;68(11):1356-76. doi: 10.1093/gerona/glt019. Epub 2013 Mar 22. J Gerontol A Biol Sci Med Sci. 2013. PMID: 23525482
-
New insights into thiol-mediated regulation of adiponectin secretion.Nutr Rev. 2008 Nov;66(11):642-5. doi: 10.1111/j.1753-4887.2008.00115.x. Nutr Rev. 2008. PMID: 19019026 Review.
-
Post-translational modifications of adiponectin: mechanisms and functional implications.Biochem J. 2008 Feb 1;409(3):623-33. doi: 10.1042/BJ20071492. Biochem J. 2008. PMID: 18177270 Review.
Cited by
-
The multi-level action of fatty acids on adiponectin production by fat cells.PLoS One. 2011;6(11):e28146. doi: 10.1371/journal.pone.0028146. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140527 Free PMC article.
-
The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging.Front Physiol. 2019 Sep 12;10:1173. doi: 10.3389/fphys.2019.01173. eCollection 2019. Front Physiol. 2019. PMID: 31572218 Free PMC article. Review.
-
Pulmonary Hypertension and Obesity: Focus on Adiponectin.Int J Mol Sci. 2019 Feb 20;20(4):912. doi: 10.3390/ijms20040912. Int J Mol Sci. 2019. PMID: 30791536 Free PMC article. Review.
-
Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease.Int J Mol Sci. 2019 May 22;20(10):2519. doi: 10.3390/ijms20102519. Int J Mol Sci. 2019. PMID: 31121868 Free PMC article. Review.
-
Regulation of adiponectin multimerization, signaling and function.Best Pract Res Clin Endocrinol Metab. 2014 Jan;28(1):25-31. doi: 10.1016/j.beem.2013.06.003. Epub 2013 Jul 5. Best Pract Res Clin Endocrinol Metab. 2014. PMID: 24417943 Free PMC article. Review.
References
-
- Bordone, L., and L. Guarente. 2005. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 6:298-305. - PubMed
-
- Brewer, J. W., and R. B. Corley. 1996. Quality control in protein biogenesis: thiol-mediated retention monitors the redox state of proteins in the endoplasmic reticulum. J. Cell Sci. 109:2383-2392. - PubMed
-
- Cabibbo, A., M. Pagani, M. Fabbri, M. Rocchi, M. R. Farmery, N. J. Bulleid, and R. Sitia. 2000. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 275:4827-4833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
