Yeast Rpb9 plays an important role in ubiquitylation and degradation of Rpb1 in response to UV-induced DNA damage
- PMID: 17452455
- PMCID: PMC1951484
- DOI: 10.1128/MCB.00404-07
Yeast Rpb9 plays an important role in ubiquitylation and degradation of Rpb1 in response to UV-induced DNA damage
Abstract
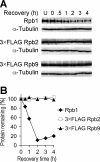
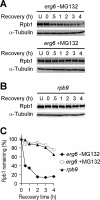
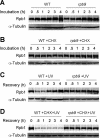
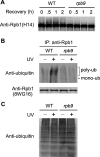
Rpb9, a nonessential subunit of RNA polymerase II (Pol II), has multiple transcription-related functions in Saccharomyces cerevisiae, including transcription elongation and transcription-coupled repair (TCR). Here we show that, in response to UV radiation, Rpb9 also functions in promoting ubiquitylation and degradation of Rpb1, the largest subunit of Pol II. This function of Rpb9 is not affected by any pathways of nucleotide excision repair, including TCR mediated by Rpb9 itself and by Rad26. Rpb9 is composed of three distinct domains: the N-terminal Zn1, the C-terminal Zn2, and the central linker. The Zn2 domain, which is dispensable for transcription elongation and TCR functions, is essential for Rpb9 to promote Rpb1 degradation, whereas the Zn1 and linker domains, which are essential for transcription elongation and TCR functions, play a subsidiary role in Rpb1 degradation. Coimmunoprecipitation analysis suggests that almost the full length of Rpb9 is required for a strong interaction with the core Pol II: deletion of the Zn2 domain causes dramatically weakened interaction, whereas deletion of Zn1 and the linker resulted in undetectable interaction. Furthermore, we show that Rpb1, rather than the whole Pol II complex, is degraded in response to UV radiation and that the degradation is primarily mediated by the 26S proteasome.
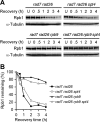
Figures



Similar articles
-
Evidence that the transcription elongation function of Rpb9 is involved in transcription-coupled DNA repair in Saccharomyces cerevisiae.Mol Cell Biol. 2006 Dec;26(24):9430-41. doi: 10.1128/MCB.01656-06. Epub 2006 Oct 9. Mol Cell Biol. 2006. PMID: 17030604 Free PMC article.
-
Rpb1 sumoylation in response to UV radiation or transcriptional impairment in yeast.PLoS One. 2009;4(4):e5267. doi: 10.1371/journal.pone.0005267. Epub 2009 Apr 22. PLoS One. 2009. PMID: 19384408 Free PMC article.
-
RNA Polymerase II Trigger Loop Mobility: INDIRECT EFFECTS OF Rpb9.J Biol Chem. 2016 Jul 8;291(28):14883-95. doi: 10.1074/jbc.M116.714394. Epub 2016 May 18. J Biol Chem. 2016. PMID: 27226557 Free PMC article.
-
Facilitators and Repressors of Transcription-coupled DNA Repair in Saccharomyces cerevisiae.Photochem Photobiol. 2017 Jan;93(1):259-267. doi: 10.1111/php.12655. Epub 2016 Nov 30. Photochem Photobiol. 2017. PMID: 27796045 Review.
-
Transcription coupled nucleotide excision repair in the yeast Saccharomyces cerevisiae: The ambiguous role of Rad26.DNA Repair (Amst). 2015 Dec;36:43-48. doi: 10.1016/j.dnarep.2015.09.006. Epub 2015 Sep 10. DNA Repair (Amst). 2015. PMID: 26429063 Review.
Cited by
-
Role of p97/VCP (Cdc48) in genome stability.Front Genet. 2013 Apr 30;4:60. doi: 10.3389/fgene.2013.00060. eCollection 2013. Front Genet. 2013. PMID: 23641252 Free PMC article.
-
Rpb9-deficient cells are defective in DNA damage response and require histone H3 acetylation for survival.Sci Rep. 2018 Feb 13;8(1):2949. doi: 10.1038/s41598-018-21110-9. Sci Rep. 2018. PMID: 29440683 Free PMC article.
-
RNAPII Degradation Factor Def1 Is Required for Development, Stress Response, and Full Virulence of Magnaporthe oryzae.J Fungi (Basel). 2023 Apr 13;9(4):467. doi: 10.3390/jof9040467. J Fungi (Basel). 2023. PMID: 37108921 Free PMC article.
-
Tfb5 is partially dispensable for Rad26 mediated transcription coupled nucleotide excision repair in yeast.DNA Repair (Amst). 2007 Nov;6(11):1661-9. doi: 10.1016/j.dnarep.2007.06.001. Epub 2007 Jul 20. DNA Repair (Amst). 2007. PMID: 17644494 Free PMC article.
-
Transcription bypass of DNA lesions enhances cell survival but attenuates transcription coupled DNA repair.Nucleic Acids Res. 2014 Dec 1;42(21):13242-53. doi: 10.1093/nar/gku1150. Epub 2014 Nov 11. Nucleic Acids Res. 2014. PMID: 25389266 Free PMC article.
References
-
- Bergmann, E., and J. M. Egly. 2001. Trichothiodystrophy, a transcription syndrome. Trends Genet. 17:279-286. - PubMed
-
- Cramer, P. 2002. Multisubunit RNA polymerases. Curr. Opin. Struct. Biol. 12:89-97. - PubMed
-
- Cramer, P., D. A. Bushnell, and R. D. Kornberg. 2001. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292:1863-1876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
