Use of a genetically defined double mutant strain of Bordetella bronchiseptica lacking adenylate cyclase and type III secretion as a live vaccine
- PMID: 17452472
- PMCID: PMC1932943
- DOI: 10.1128/IAI.01648-06
Use of a genetically defined double mutant strain of Bordetella bronchiseptica lacking adenylate cyclase and type III secretion as a live vaccine
Abstract
While most vaccines consisting of killed bacteria induce high serum antibody titers, they do not always confer protection as effective as that induced by infection, particularly against mucosal pathogens. Bordetella bronchiseptica is a gram-negative respiratory pathogen that is endemic in many nonhuman mammalian populations and causes substantial disease in a variety of animals. At least 14 different live attenuated vaccines against this pathogen are available for use in a variety of livestock and companion animals. However, there are few published data on the makeup or efficacy of these vaccines. Here we report the use of a genetically engineered double mutant of B. bronchiseptica, which lacks adenylate cyclase and type III secretion, as a vaccine candidate. This strain is safe at high doses, even for highly immunocompromised animals, and induces immune responses that are protective against highly divergent B. bronchiseptica strains, preventing colonization in the lower respiratory tract and decreasing the bacterial burden in the upper respiratory tract. This novel B. bronchiseptica vaccine candidate induces strong local immunity while eliminating damage caused by the two predominant cytotoxic mechanisms.
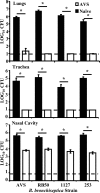
Figures







Similar articles
-
Membrane Vesicles Derived from Bordetella bronchiseptica: Active Constituent of a New Vaccine against Infections Caused by This Pathogen.Appl Environ Microbiol. 2018 Jan 31;84(4):e01877-17. doi: 10.1128/AEM.01877-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29180369 Free PMC article.
-
Cross-species protection mediated by a Bordetella bronchiseptica strain lacking antigenic homologs present in acellular pertussis vaccines.Infect Immun. 2010 May;78(5):2008-16. doi: 10.1128/IAI.01142-09. Epub 2010 Feb 22. Infect Immun. 2010. PMID: 20176797 Free PMC article.
-
Dual mechanism of protection by live attenuated Bordetella pertussis BPZE1 against Bordetella bronchiseptica in mice.Vaccine. 2012 Aug 31;30(40):5864-70. doi: 10.1016/j.vaccine.2012.07.005. Epub 2012 Jul 17. Vaccine. 2012. PMID: 22814407
-
Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens.FEMS Immunol Med Microbiol. 2003 Jul 15;37(2-3):121-8. doi: 10.1016/S0928-8244(03)00068-3. FEMS Immunol Med Microbiol. 2003. PMID: 12832115 Review.
-
Bordetella bronchiseptica and Bordetella pertussis: Similarities and Differences in Infection, Immuno-Modulation, and Vaccine Considerations.Clin Microbiol Rev. 2023 Sep 21;36(3):e0016422. doi: 10.1128/cmr.00164-22. Epub 2023 Jun 12. Clin Microbiol Rev. 2023. PMID: 37306571 Free PMC article. Review.
Cited by
-
Comparative role of immunoglobulin A in protective immunity against the Bordetellae.Infect Immun. 2007 Sep;75(9):4416-22. doi: 10.1128/IAI.00412-07. Epub 2007 Jun 25. Infect Immun. 2007. PMID: 17591791 Free PMC article.
-
A Marker-Free Bordetella bronchiseptica aroA/bscN Double Deleted Mutant Confers Protection Against Lethal Challenge.Vaccines (Basel). 2019 Nov 4;7(4):176. doi: 10.3390/vaccines7040176. Vaccines (Basel). 2019. PMID: 31690029 Free PMC article.
-
Active and passive immunizations with Bordetella colonization factor A protect mice against respiratory challenge with Bordetella bronchiseptica.Infect Immun. 2009 Feb;77(2):885-95. doi: 10.1128/IAI.01076-08. Epub 2008 Dec 8. Infect Immun. 2009. PMID: 19064638 Free PMC article.
-
Subcutaneous Immunization of Dogs With Bordetella bronchiseptica Bacterial Ghost Vaccine.Front Immunol. 2019 Jun 25;10:1377. doi: 10.3389/fimmu.2019.01377. eCollection 2019. Front Immunol. 2019. PMID: 31293571 Free PMC article.
-
Mucosal Immunization Against Pertussis: Lessons From the Past and Perspectives.Front Immunol. 2021 Jun 15;12:701285. doi: 10.3389/fimmu.2021.701285. eCollection 2021. Front Immunol. 2021. PMID: 34211481 Free PMC article.
References
-
- Bey, R. F., F. J. Shade, R. A. Goodnow, and R. C. Johnson. 1981. Intranasal vaccination of dogs with liver avirulent Bordetella bronchiseptica: correlation of serum agglutination titer and the formation of secretory IgA with protection against experimentally induced infectious tracheobronchitis. Am. J. Vet. Res. 42:1130-1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

