Adapter protein SH2-Bbeta stimulates actin-based motility of Listeria monocytogenes in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion
- PMID: 17452473
- PMCID: PMC1932951
- DOI: 10.1128/IAI.00214-07
Adapter protein SH2-Bbeta stimulates actin-based motility of Listeria monocytogenes in a vasodilator-stimulated phosphoprotein (VASP)-dependent fashion
Abstract
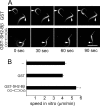
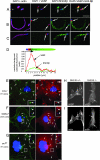
SH2-Bbeta (Src homology 2 Bbeta) is an adapter protein that is required for maximal growth hormone-dependent actin reorganization in membrane ruffling and cell motility. Here we show that SH2-Bbeta is also required for maximal actin-based motility of Listeria monocytogenes. SH2-Bbeta localizes to Listeria-induced actin tails and increases the rate of bacterial propulsion in infected cells and in cell extracts. Furthermore, Listeria motility is decreased in mouse embryo fibroblasts from SH2-B(-/-) mice. Both recruitment of SH2-Bbeta to Listeria and SH2-Bbeta stimulation of actin-based propulsion require the vasodilator-stimulated phosphoprotein (VASP), which binds ActA at the surfaces of Listeria cells and enhances bacterial actin-based motility. SH2-Bbeta enhances actin-based movement of ActA-coated beads in a biomimetic actin-based motility assay, provided that VASP is present. In vitro binding assays show that SH2-Bbeta binds ActA but not VASP; however, binding to ActA is greater in the presence of VASP. Because VASP also plays an essential regulatory role in actin-based processes in eukaryotic cells, the present results provide mechanistic insight into the functions of both SH2-Bbeta and VASP in motility and also increase our understanding of the fundamental mechanism by which Listeria spreads.
Figures





Similar articles
-
Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility.J Cell Biol. 2001 Oct 1;155(1):89-100. doi: 10.1083/jcb.200106061. J Cell Biol. 2001. PMID: 11581288 Free PMC article.
-
Identification of cofilin, coronin, Rac and capZ in actin tails using a Listeria affinity approach.J Cell Sci. 1998 Oct;111 ( Pt 19):2877-84. doi: 10.1242/jcs.111.19.2877. J Cell Sci. 1998. PMID: 9730980
-
Ena/VASP proteins contribute to Listeria monocytogenes pathogenesis by controlling temporal and spatial persistence of bacterial actin-based motility.Mol Microbiol. 2003 Sep;49(5):1361-75. doi: 10.1046/j.1365-2958.2003.03639.x. Mol Microbiol. 2003. PMID: 12940993
-
The focal adhesion phosphoprotein, VASP.Int J Biochem Cell Biol. 1998 Mar;30(3):307-11. doi: 10.1016/s1357-2725(97)00101-5. Int J Biochem Cell Biol. 1998. PMID: 9611773 Review.
-
Actin-based motility: stop and go with Ena/VASP proteins.Trends Biochem Sci. 2001 Apr;26(4):243-9. doi: 10.1016/s0968-0004(00)01785-0. Trends Biochem Sci. 2001. PMID: 11295557 Review.
Cited by
-
Phosphorylation of the adaptor protein SH2B1β regulates its ability to enhance growth hormone-dependent macrophage motility.J Cell Sci. 2013 Apr 15;126(Pt 8):1733-43. doi: 10.1242/jcs.113050. Epub 2013 Feb 26. J Cell Sci. 2013. PMID: 23444381 Free PMC article.
-
The nucleolar δ isoform of adapter protein SH2B1 enhances morphological complexity and function of cultured neurons.J Cell Sci. 2022 Feb 1;135(3):jcs259179. doi: 10.1242/jcs.259179. Epub 2022 Feb 10. J Cell Sci. 2022. PMID: 35019135 Free PMC article.
-
Adapter protein SH2B1beta binds filamin A to regulate prolactin-dependent cytoskeletal reorganization and cell motility.Mol Endocrinol. 2011 Jul;25(7):1231-43. doi: 10.1210/me.2011-0056. Epub 2011 May 12. Mol Endocrinol. 2011. PMID: 21566085 Free PMC article.
-
RECON-Dependent Inflammation in Hepatocytes Enhances Listeria monocytogenes Cell-to-Cell Spread.mBio. 2018 May 15;9(3):e00526-18. doi: 10.1128/mBio.00526-18. mBio. 2018. PMID: 29764944 Free PMC article.
-
Endogenous SH2B1 protein localizes to lamellipodia and filopodia: platinum replica electron-microscopy study.MicroPubl Biol. 2025 Jan 17;2025:10.17912/micropub.biology.001451. doi: 10.17912/micropub.biology.001451. eCollection 2025. MicroPubl Biol. 2025. PMID: 39897164 Free PMC article.
References
-
- Bear, J. E., J. J. Loureiro, I. Libova, R. Fassler, J. Wehland, and F. B. Gertler. 2000. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101:717-728. - PubMed
-
- Bear, J. E., T. M. Svitkina, M. Krause, D. A. Schafer, J. J. Loureiro, G. A. Strasser, I. V. Maly, O. Y. Chaga, J. A. Cooper, G. G. Borisy, and F. B. Gertler. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109:509-521. - PubMed
-
- Boujemaa-Paterski, R., E. Gouin, G. Hansen, S. Samarin, C. Le Clainche, D. Didry, P. Dehoux, P. Cossart, C. Kocks, M. F. Carlier, and D. Pantaloni. 2001. Listeria protein ActA mimics WASp family proteins: it activates filament barbed end branching by Arp2/3 complex. Biochemistry 40:11390-11404. - PubMed
-
- Cicchetti, G., P. Maurer, P. Wagener, and C. Kocks. 1999. Actin and phosphoinositide binding by the ActA protein of the bacterial pathogen Listeria monocytogenes. J. Biol. Chem. 274:33616-33626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

