Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model
- PMID: 17452485
- PMCID: PMC1913249
- DOI: 10.1128/AAC.00147-07
Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model
Abstract
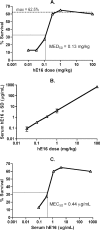
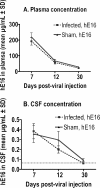
A potent anti-West Nile virus (anti-WNV)-neutralizing humanized monoclonal antibody, hE16, was previously shown to improve the survival of WNV-infected hamsters when it was administered intraperitoneally (i.p.), even after the virus had infected neurons in the brain. In this study, we evaluated the therapeutic limit of hE16 for the treatment of WNV infection in hamsters by comparing single-dose peripheral (i.p.) therapy with direct administration into the pons through a convection-enhanced delivery (CED) system. At day 5 after infection, treatments with hE16 by the peripheral and the CED routes were equally effective at reducing morbidity and mortality. In contrast, at day 6 only the treatment by the CED route protected the hamsters from lethal infection. These experiments suggest that hE16 can directly control WNV infection in the central nervous system. In support of this, hE16 administered i.p. was detected in a time-dependent manner in the serum, cerebrospinal fluid (CSF), cerebral cortex, brain stem, and spinal cord in CSF. A linear relationship between the hE16 dose and the concentration in serum was observed, and maximal therapeutic activity occurred at doses of 0.32 mg/kg of body weight or higher, which produced serum hE16 concentrations of 1.3 microg/ml or higher. Overall, these data suggest that in hamsters hE16 can ameliorate neurological disease after significant viral replication has occurred, although there is a time window that limits therapeutic efficacy.
Figures

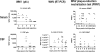

Similar articles
-
Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters.J Infect Dis. 2006 Nov 1;194(9):1300-8. doi: 10.1086/508293. Epub 2006 Sep 22. J Infect Dis. 2006. PMID: 17041857
-
West Nile virus-induced acute flaccid paralysis is prevented by monoclonal antibody treatment when administered after infection of spinal cord neurons.J Neurovirol. 2008 Apr;14(2):152-63. doi: 10.1080/13550280801958930. J Neurovirol. 2008. PMID: 18444087 Free PMC article.
-
Treatment of spatial memory impairment in hamsters infected with West Nile virus using a humanized monoclonal antibody MGAWN1.Antiviral Res. 2011 Jul;91(1):43-9. doi: 10.1016/j.antiviral.2011.04.011. Epub 2011 Apr 30. Antiviral Res. 2011. PMID: 21554903 Free PMC article.
-
The molecular basis of antibody protection against West Nile virus.Curr Top Microbiol Immunol. 2008;317:125-53. doi: 10.1007/978-3-540-72146-8_5. Curr Top Microbiol Immunol. 2008. PMID: 17990792 Review.
-
Mapping and analysis of West Nile virus-specific monoclonal antibodies: prospects for vaccine development.Expert Rev Vaccines. 2007 Apr;6(2):183-91. doi: 10.1586/14760584.6.2.183. Expert Rev Vaccines. 2007. PMID: 17408368 Review.
Cited by
-
Genotype-specific neutralization and protection by antibodies against dengue virus type 3.J Virol. 2010 Oct;84(20):10630-43. doi: 10.1128/JVI.01190-10. Epub 2010 Aug 11. J Virol. 2010. PMID: 20702644 Free PMC article.
-
West Nile virus drug discovery.Viruses. 2013 Dec 3;5(12):2977-3006. doi: 10.3390/v5122977. Viruses. 2013. PMID: 24300672 Free PMC article. Review.
-
A game of numbers: the stoichiometry of antibody-mediated neutralization of flavivirus infection.Prog Mol Biol Transl Sci. 2015;129:141-66. doi: 10.1016/bs.pmbts.2014.10.005. Epub 2014 Dec 1. Prog Mol Biol Transl Sci. 2015. PMID: 25595803 Free PMC article. Review.
-
A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes.PLoS Pathog. 2009 May;5(5):e1000453. doi: 10.1371/journal.ppat.1000453. Epub 2009 May 29. PLoS Pathog. 2009. PMID: 19478866 Free PMC article.
-
The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1.PLoS Pathog. 2010 Apr 1;6(4):e1000823. doi: 10.1371/journal.ppat.1000823. PLoS Pathog. 2010. PMID: 20369024 Free PMC article.
References
-
- Banks, M., G. S. Heath, S. S. Grierson, D. P. King, A. Gresham, R. Girones, F. Widen, and T. J. Harrison. 2004. Evidence for the presence of hepatitis E virus in pigs in the United Kingdom. Vet. Rec. 154223-227. - PubMed
-
- Ben-Nathan, D., S. Lustig, G. Tam, S. Robinzon, S. Segal, and B. Rager-Zisman. 2003. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect. Dis. 1885-12. - PubMed
-
- Boeckh, M., M. M. Berrey, R. A. Bowden, S. W. Crawford, J. Balsley, and L. Corey. 2001. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J. Infect. Dis. 184350-354. - PubMed
-
- Datta-Mannan, A., D. R. Witcher, Y. Tang, J. Watkins, and V. J. Wroblewski. 2007. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J. Biol. Chem. 2821709-1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

