PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization
- PMID: 17452534
- PMCID: PMC2064142
- DOI: 10.1083/jcb.200611011
PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization
Abstract
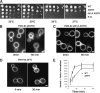
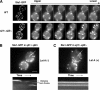
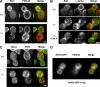
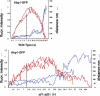
The lipid phosphatidylinositol-4,5-bisphosphate (PtdIns[4,5]P(2)) appears to play an important role in endocytosis. However, the timing of its formation and turnover, and its specific functions at different stages during endocytic internalization, have not been established. In this study, Sla2 ANTH-GFP and Sjl2-3GFP were expressed as functional fusion proteins at endogenous levels to quantitatively explore PtdIns(4,5)P(2) dynamics during endocytosis in yeast. Our results indicate that PtdIns(4,5)P(2) levels increase and decline in conjunction with coat and actin assembly and disassembly, respectively. Live-cell image analysis of endocytic protein dynamics in an sjl1Delta sjl2Delta mutant, which has elevated PtdIns(4,5)P(2) levels, revealed that the endocytic machinery is still able to assemble and disassemble dynamically, albeit nonproductively. The defects in the dynamic behavior of the various endocytic proteins in this double mutant suggest that PtdIns(4,5)P(2) turnover is required for multiple stages during endocytic vesicle formation. Furthermore, our results indicate that PtdIns(4,5)P(2) turnover may act in coordination with the Ark1/Prk1 protein kinases in stimulating disassembly of the endocytic machinery.
Figures





References
-
- Aguilar, R.C., H.A. Watson, and B. Wendland. 2003. The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278:10737–10743. - PubMed
-
- Bairstow, S.F., K. Ling, X. Su, A.J. Firestone, C. Carbonara, and R.A. Anderson. 2006. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 281:20632–20642. - PubMed
-
- Cremona, O., G. Di Paolo, M.R. Wenk, A. Luthi, W.T. Kim, K. Takei, L. Daniell, Y. Nemoto, S.B. Shears, R.A. Flavell, et al. 1999. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 99:179–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

