Computational network model prediction of hemodynamic alterations due to arteriolar remodeling in interval sprint trained skeletal muscle
- PMID: 17454671
- PMCID: PMC2662752
- DOI: 10.1080/10739680601139237
Computational network model prediction of hemodynamic alterations due to arteriolar remodeling in interval sprint trained skeletal muscle
Abstract
Objectives: Exercise training is known to enhance skeletal muscle blood flow capacity, with high-intensity interval sprint training (IST) primarily affecting muscles with a high proportion of fast twitch glycolytic fibers. The objective of this study was to determine the relative contributions of new arteriole formation and lumenal arteriolar remodeling to enhanced flow capacity and the impact of these adaptations on local microvascular hemodynamics deep within the muscle.
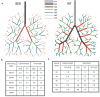
Methods: The authors studied arteriolar adaptation in the white/mixed-fiber portion of gastrocnemius muscles of IST (6 bouts of running/day; 2.5 min/bout; 60 m/min speed; 15% grade; 4.5 min rest between bouts; 5 training days/wk; 10 wks total) and sedentary (SED) control rats using whole-muscle Microfil casts. Dimensional and topological data were then used to construct a series of computational hemodynamic network models that incorporated physiological red blood cell distributions and hematocrit and diameter dependent apparent viscosities.
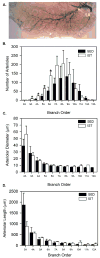
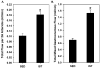
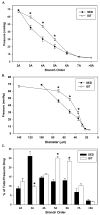
Results: In comparison to SED controls, IST elicited a significant increase in arterioles/order in the 3A through 6A generations. Predicted IST and SED flows through the 2A generation agreed closely with in vivo measurements made in a previous study, illustrating the accuracy of the model. IST shifted the bulk of the pressure drop across the network from the 3As to the 4As and 5As, and flow capacity increased from 0.7 mL/min in SED to 1.5 mL/min in IST when a driving pressure of 80 mmHg was applied.
Conclusions: The primary adaptation to IST is an increase in arterioles in the 3A through 6A generations, which, in turn, creates an approximate doubling of flow capacity and a deeper penetration of high pressure into the arteriolar network.
Figures
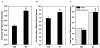
References
-
- Adair TH, Gay WJ, Montani J-P. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol. 1990;259:393–404. - PubMed
-
- Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol Heart Circ Physiol. 1984;246:H59–H68. - PubMed
-
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. - PubMed
-
- Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW., Jr Microvascular rarefaction and tissue vascular resistance in hypertension. Am J Physiol Heart Circ Physiol. 1989;256:H126–H131. - PubMed
-
- Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurance-trained rats. J Appl Physiol. 1996;81:619–626. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

