Expression of insulin-like growth factor system genes during the early postnatal neurogenesis in the mouse hippocampus
- PMID: 17455296
- PMCID: PMC2302789
- DOI: 10.1002/jnr.21289
Expression of insulin-like growth factor system genes during the early postnatal neurogenesis in the mouse hippocampus
Abstract
Insulin-like growth factor-1 (IGF-1) is essential to hippocampal neurogenesis and the neuronal response to hypoxia/ischemia injury. IGF (IGF-1 and -2) signaling is mediated primarily by the type 1 IGF receptor (IGF-1R) and modulated by six high-affinity binding proteins (IGFBP) and the type 2 IGF receptor (IGF-2R), collectively termed IGF system proteins. Defining the precise cells that express each is essential to understanding their roles. With the exception of IGFBP-1, we found that mouse hippocampus expresses mRNA for each of these proteins during the first 2 weeks of postnatal life. Compared to postnatal day 14 (P14), mRNA abundance at P5 was higher for IGF-1, IGFBP-2, -3, and -5 (by 71%, 108%, 100%, and 98%, respectively), lower for IGF-2, IGF-2R, and IGFBP-6 (by 65%, 78%, and 44%, respectively), and unchanged for IGF-1R and IGFBP-4. Using laser capture microdissection (LCM), we found that granule neurons and pyramidal neurons exhibited identical patterns of expression of IGF-1, IGF-1R, IGF-2R, IGFBP-2, and -4, but did not express other IGF system genes. We then compared IGF system expression in mature granule neurons and their progenitors. Progenitors exhibited higher mRNA levels of IGF-1 and IGF-1R (by 130% and 86%, respectively), lower levels of IGF-2R (by 72%), and similar levels of IGFBP-4. Our data support a role for IGF in hippocampal neurogenesis and provide evidence that IGF actions are regulated within a defined in vivo milieu.
Copyright (c) 2007 Wiley-Liss, Inc.
Figures


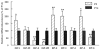


Similar articles
-
mRNA expression patterns of insulin-like growth factor system components in human neuroendocrine tumours.Eur J Clin Invest. 2000 Aug;30(8):729-39. doi: 10.1046/j.1365-2362.2000.00700.x. Eur J Clin Invest. 2000. PMID: 10964166
-
Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic-ischemic injury.Brain Res Mol Brain Res. 1998 Aug 31;59(2):119-34. doi: 10.1016/s0169-328x(98)00122-3. Brain Res Mol Brain Res. 1998. PMID: 9729323
-
Brain Insulin-Like Growth Factor-I Directs the Transition from Stem Cells to Mature Neurons During Postnatal/Adult Hippocampal Neurogenesis.Stem Cells. 2016 Aug;34(8):2194-209. doi: 10.1002/stem.2397. Epub 2016 May 27. Stem Cells. 2016. PMID: 27144663
-
Insulin-like growth factors and insulin-like growth factor binding proteins in the endometrium. Effect of intrauterine levonorgestrel delivery.Hum Reprod. 2000 Aug;15 Suppl 3:173-81. doi: 10.1093/humrep/15.suppl_3.173. Hum Reprod. 2000. PMID: 11041233 Review.
-
Nuclear localization and actions of the insulin-like growth factor 1 (IGF-1) system components: Transcriptional regulation and DNA damage response.Mutat Res Rev Mutat Res. 2020 Apr-Jun;784:108307. doi: 10.1016/j.mrrev.2020.108307. Epub 2020 Feb 27. Mutat Res Rev Mutat Res. 2020. PMID: 32430099 Review.
Cited by
-
The metabolic effects of resumption of a high fat diet after weight loss are sex dependent in mice.Sci Rep. 2023 Aug 14;13(1):13227. doi: 10.1038/s41598-023-40514-w. Sci Rep. 2023. PMID: 37580448 Free PMC article.
-
The Role of Induced Pluripotent Stem Cells in the Treatment of Stroke.Curr Neuropharmacol. 2024;22(14):2368-2383. doi: 10.2174/1570159X22666240603084558. Curr Neuropharmacol. 2024. PMID: 39403058 Free PMC article. Review.
-
Protective effects of berberine against diabetes-associated cognitive decline in mice.Acta Diabetol. 2025 Jun;62(6):943-955. doi: 10.1007/s00592-024-02411-0. Epub 2024 Nov 8. Acta Diabetol. 2025. PMID: 39514003
-
Age-related changes in energy metabolism in peripheral mononuclear blood cells (PBMCs) and the brains of cognitively healthy seniors.Geroscience. 2024 Feb;46(1):981-998. doi: 10.1007/s11357-023-00810-9. Epub 2023 Jun 13. Geroscience. 2024. PMID: 37308768 Free PMC article.
-
Gender differences and lateralization in the distribution pattern of insulin-like growth factor-1 receptor in developing rat hippocampus: an immunohistochemical study.Cell Mol Neurobiol. 2014 Mar;34(2):215-26. doi: 10.1007/s10571-013-0005-x. Epub 2013 Nov 28. Cell Mol Neurobiol. 2014. PMID: 24287499 Free PMC article.
References
-
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. - PubMed
-
- Aguado F, Rodrigo J, Cacicedo L, Mellstrom B. Distribution of insulin-like growth factor-I receptor mRNA in rat brain. Regulation in the hypothalamo-neurohypophysial system. J Mol Endocrinol. 1993;11:231–239. - PubMed
-
- Ahmad AM, Burns J, Gardner R, Graham C. Delayed and disturbed morphogenesis of the umbilical blood vessels in insulin-like growth factor-II deficient conceptuses (Igf2m+/p−) Dev Dyn. 2005;233:88–94. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

