Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells
- PMID: 17456723
- PMCID: PMC2629730
- DOI: 10.1182/blood-2006-12-060814
Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells
Abstract
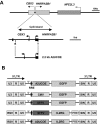
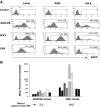
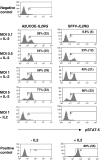
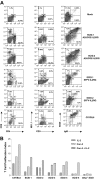
Ubiquitously acting chromatin opening elements (UCOEs) consist of methylation-free CpG islands encompassing dual divergently transcribed promoters of housekeeping genes that have been shown to confer resistance to transcriptional silencing and to produce consistent and stable transgene expression in tissue culture systems. To develop improved strategies for hematopoietic cell gene therapy, we have assessed the potential of the novel human HNRPA2B1-CBX3 UCOE (A2UCOE) within the context of a self-inactivating (SIN) lentiviral vector. Unlike viral promoters, the enhancer-less A2UCOE gave rise to populations of cells that expressed a reporter transgene at a highly reproducible level. The efficiency of expression per vector genome was also markedly increased in vivo compared with vectors incorporating either spleen focus-forming virus (SFFV) or cytomegalovirus (CMV) promoters, suggesting a relative resistance to silencing. Furthermore, an A2UCOE-IL2RG vector fully restored the IL-2 signaling pathway within IL2RG-deficient human cells in vitro and successfully rescued the X-linked severe combined immunodeficiency (SCID-X1) phenotype in a mouse model of this disease. These data indicate that the A2UCOE displays highly reliable transcriptional activity within a lentiviral vector, largely overcoming insertion-site position effects and giving rise to therapeutically relevant levels of gene expression. These properties are achieved in the absence of classic enhancer activity and therefore may confer a high safety profile.
Figures



Similar articles
-
Physiological regulation of transgene expression by a lentiviral vector containing the A2UCOE linked to a myeloid promoter.Gene Ther. 2012 Oct;19(10):1018-29. doi: 10.1038/gt.2011.167. Epub 2011 Nov 10. Gene Ther. 2012. PMID: 22071971
-
Long-term reproducible expression in human fetal liver hematopoietic stem cells with a UCOE-based lentiviral vector.PLoS One. 2014 Aug 12;9(8):e104805. doi: 10.1371/journal.pone.0104805. eCollection 2014. PLoS One. 2014. PMID: 25118036 Free PMC article.
-
A ubiquitous chromatin opening element (UCOE) confers resistance to DNA methylation-mediated silencing of lentiviral vectors.Mol Ther. 2010 Sep;18(9):1640-9. doi: 10.1038/mt.2010.132. Epub 2010 Jun 29. Mol Ther. 2010. PMID: 20588258 Free PMC article.
-
Ubiquitous Chromatin-opening Elements (UCOEs): Applications in biomanufacturing and gene therapy.Biotechnol Adv. 2017 Sep;35(5):557-564. doi: 10.1016/j.biotechadv.2017.05.004. Epub 2017 May 17. Biotechnol Adv. 2017. PMID: 28528197 Review.
-
Gene therapy for PIDs: progress, pitfalls and prospects.Gene. 2013 Aug 10;525(2):174-81. doi: 10.1016/j.gene.2013.03.098. Epub 2013 Apr 6. Gene. 2013. PMID: 23566838 Free PMC article. Review.
Cited by
-
Recent advances in lentiviral vector development and applications.Mol Ther. 2010 Mar;18(3):477-90. doi: 10.1038/mt.2009.319. Epub 2010 Jan 19. Mol Ther. 2010. PMID: 20087315 Free PMC article. Review.
-
New lines of GFP transgenic rats relevant for regenerative medicine and gene therapy.Transgenic Res. 2010 Oct;19(5):745-63. doi: 10.1007/s11248-009-9352-2. Epub 2010 Jan 22. Transgenic Res. 2010. PMID: 20094912
-
Applications of 3D Bioprinting Technology in Induced Pluripotent Stem Cells-Based Tissue Engineering.Micromachines (Basel). 2022 Jan 20;13(2):155. doi: 10.3390/mi13020155. Micromachines (Basel). 2022. PMID: 35208280 Free PMC article. Review.
-
Safer, silencing-resistant lentiviral vectors: optimization of the ubiquitous chromatin-opening element through elimination of aberrant splicing.J Virol. 2012 Sep;86(17):9088-95. doi: 10.1128/JVI.00485-12. Epub 2012 Jun 13. J Virol. 2012. PMID: 22696657 Free PMC article.
-
Effective, safe, and sustained correction of murine XLA using a UCOE-BTK promoter-based lentiviral vector.Mol Ther Methods Clin Dev. 2021 Jan 20;20:635-651. doi: 10.1016/j.omtm.2021.01.007. eCollection 2021 Mar 12. Mol Ther Methods Clin Dev. 2021. PMID: 33718514 Free PMC article.
References
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint BG, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. - PubMed
-
- Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
-
- Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. - PubMed
-
- Gaspar HB, Bjorkegren E, Parsley K, et al. Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol Ther. 2006;14:505–513. - PubMed
-
- Schmidt M, Hacein-Bey-Abina S, Wissler M, et al. Clonal evidence for the transduction of CD34(+) cells with lymphomyeloid differentiation potential and self-renewal capacity in the SCID-X1 gene therapy trial. Blood. 2005;105:2699–2706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

