Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles
- PMID: 17456772
- PMCID: PMC1854961
- DOI: 10.2353/ajpath.2007.061178
Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles
Abstract
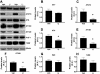
Glycogen synthase kinase 3 (GSK-3) is a major kinase implicated in the pathogenesis of Alzheimer's disease (AD), and reducing its activity may have therapeutic efficacy. Two variants exist, referred to as GSK-3 alpha and GSK-3beta. In addition to the latter's well-described role in the phosphorylation of tau, reports also suggest that GSK-3 alpha may regulate amyloid precursor protein processing and Abeta formation. The activities of both GSK-3 alpha and GSK-3beta are reduced by lithium, a well-tolerated drug used in humans to combat bipolar disorder. Here, we investigate the therapeutic efficacy of chronic lithium administration in aged 3xTg-AD mice that harbor both plaques and tangles. We found that lithium reduced tau phosphorylation but did not significantly alter the A beta load. Despite the reduction in phosphotau, lithium treatment did not improve deficits in working memory. Although other studies have investigated the effects of lithium on tau biochemistry, this study represents the first to address comprehensively its therapeutic potential on other critical aspects of AD including its effect on A beta and learning and memory. It remains to be determined from human clinical trials whether lithium treatment alone will improve the clinical outcome in AD patients. These results, however, suggest that the most efficacious treatment will be combining lithium with other anti-A beta interventions.
Figures




Comment in
-
Treating the lesions, not the disease.Am J Pathol. 2007 May;170(5):1457-9. doi: 10.2353/ajpath.2007.070193. Am J Pathol. 2007. PMID: 17456753 Free PMC article. Review. No abstract available.
References
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004;10:452–458. - PubMed
-
- Sperber BR, Leight S, Goedert M, Lee VM. Glycogen synthase kinase-3 beta phosphorylates tau protein at multiple sites in intact cells. Neurosci Lett. 1995;197:149–153. - PubMed
-
- Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. - PubMed
-
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102:6990–6995. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

