The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation
- PMID: 17456781
- PMCID: PMC1854970
- DOI: 10.2353/ajpath.2007.060886
The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation
Abstract
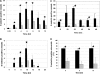
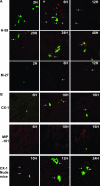
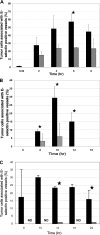
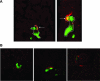
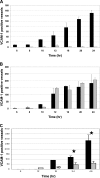
Inflammation can play a regulatory role in cancer progression and metastasis. Previously, we have shown that metastatic tumor cells entering the liver trigger a proinflammatory response involving Kupffer cell-mediated release of tumor necrosis factor-alpha and the up-regulation of vascular endothelial cell adhesion receptors, such as E-selectin. Here, we analyzed spatio-temporal aspects of the ensuing tumor-endothelial cell interaction using human colorectal carcinoma CX-1 and murine carcinoma H-59 cells and a combination of immunohistochemistry, confocal microscopy, and three-dimensional reconstruction. E-selectin expression was evident mainly on sinusoidal vessels by 6 and 10 hours, respectively, following H-59 and CX-1 inoculation, and this corresponded to a stabilization of the number of tumor cells within the sinuses. Tumor cells arrested in E-selectin(+) vessels and appeared to flatten and traverse the vessel lining, away from sites of intense E-selectin staining. This process was evident by 8 (H-59) and 12 (CX-1) hours after inoculation, coincided with increased endothelial vascular cell adhesion molecule-1 expression, and involved tumor cell attachment in areas of intense vascular cell adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 expression. Nonmetastatic (human) MIP-101 and (murine) M-27 cells induced a weaker response and could not be seen to extravasate. The results show that metastatic tumor cells can alter the hepatic microvasculature and use newly expressed endothelial cell receptors to arrest and extravasate.
Figures



References
-
- Witz IP, Levy-Nissenbaum O. The tumor microenvironment in the post-PAGET era. Cancer Lett. 2006;242:1–10. - PubMed
-
- Robinson SC, Coussens LM. Soluble mediators of inflammation during tumor development. Adv Cancer Res. 2005;93:159–187. - PubMed
-
- Fahy B, Bold RJ. Epidemiology and molecular genetics of colorectal cancer. Surg Oncol. 1998;7:115–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

