Exposure to bacterial DNA before hemorrhagic shock strongly aggravates systemic inflammation and gut barrier loss via an IFN-gamma-dependent route
- PMID: 17457174
- PMCID: PMC1877070
- DOI: 10.1097/01.sla.0000251513.59983.3b
Exposure to bacterial DNA before hemorrhagic shock strongly aggravates systemic inflammation and gut barrier loss via an IFN-gamma-dependent route
Abstract
Objective: To investigate the role of bacterial DNA in development of an excessive inflammatory response and loss of gut barrier loss following systemic hypotension.
Summary background data: Bacterial infection may contribute to development of inflammatory complications following major surgery; however, the pathogenesis is not clear. A common denominator of bacterial infection is bacterial DNA characterized by unmethylated CpG motifs. Recently, it has been shown that bacterial DNA or synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG-ODN) are immunostimulatory leading to release of inflammatory mediators.
Methods: Rats were exposed to CpG-ODN prior to a nonlethal hemorrhagic shock. The role of interferon-gamma (IFN-gamma) was investigated by administration of anti IFN-gamma antibodies.
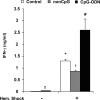
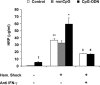
Results: Exposure to CpG-ODN prior to hemorrhagic shock significantly augmented shock-induced release of IFN-gamma, tumor necrosis factor-alpha (TNF-alpha) (P < 0.05), interleukin (IL)-6 (P < 0.05), and nitrite levels (P < 0.05), while there was a defective IL-10 response (P < 0.05). Simultaneously, expression of Toll-like receptor (TLR) 4 in the liver was markedly enhanced. Furthermore, intestinal permeability for HRP significantly increased and bacterial translocation was enhanced in hemorrhagic shock rats pretreated with CpG-ODN. Interestingly, inhibition of IFN-gamma in CpG-treated animals reduced TNF-alpha (P < 0.05), IL-6 (P < 0.05), nitrite (P < 0.05), and intestinal permeability following hemorrhagic shock (P < 0.05) and down-regulated expression of TLR4.
Conclusion: Exposure to bacterial DNA strongly aggravates the inflammatory response, disrupts the intestinal barrier, and up-regulates TLR4 expression in the liver following hemorrhagic shock. These effects are mediated via an IFN-gamma-dependent route. In the clinical setting, bacterial DNA may be important in development of inflammatory complications in surgical patients with bacterial infection.
Figures


References
-
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. - PubMed
-
- Fan J, Kapus A, Marsden PA, et al. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–5259. - PubMed
-
- Turnbull RG, Talbot JA, Hamilton SM. Hemodynamic changes and gut barrier function in sequential hemorrhagic and endotoxic shock. J Trauma. 1995;38:705–712. - PubMed
-
- Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. - PubMed
-
- Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132; quiz 133–134; discussion 96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

