Modern potentiometry
- PMID: 17457791
- PMCID: PMC2515866
- DOI: 10.1002/anie.200605068
Modern potentiometry
Abstract
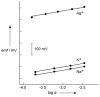
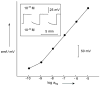
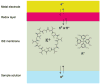
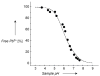
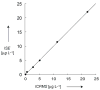
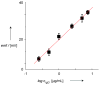
For most chemists, potentiometry with ion-selective electrodes (ISEs) primarily means pH measurements with a glass electrode. Those interested in clinical analysis might know that ISEs, routinely used for the determination of blood electrolytes, have a market size comparable to that of glass electrodes. It is even less well known that potentiometry went through a silent revolution during the past decade. The lower detection limit and the discrimination of interfering ions (the selectivity coefficients) have been improved in many cases by factors up to 10(6) and 10(10), respectively, thus allowing their application in fields such as environmental trace analysis and potentiometric biosensing. The determination of complex formation constants for lipophilic hosts and ionic guests is also covered in this Minireview.
Figures


References
-
- Young CC. J Chem Educ. 1997;74:177–182.
-
- Morf WE. The Principles of Ion–Selective Electrodes and of Membrane Transport. Elsevier; New York: 1981.
-
- Koryta J, Stulik K. Ion–Selective Electrodes. Cambridge University Press; Cambridge, GB: 1983.
-
- Buck RP, Lindner E. Anal Chem. 2001;73:88A–97A. - PubMed
-
- Bakker E, Bühlmann P, Pretsch E. Chem Rev. 1997;97:3083–3132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

