Function of longitudinal vs circular muscle fibers in esophageal peristalsis, deduced with mathematical modeling
- PMID: 17457963
- PMCID: PMC4146916
- DOI: 10.3748/wjg.v13.i9.1335
Function of longitudinal vs circular muscle fibers in esophageal peristalsis, deduced with mathematical modeling
Abstract
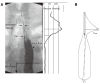
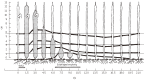
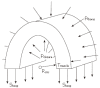
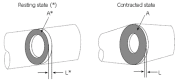
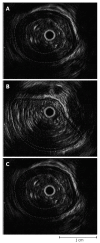
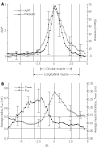
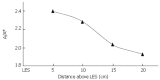
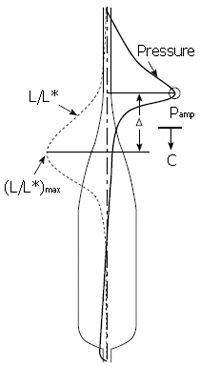
We summarize from previous works the functions of circular vs. longitudinal muscle in esophageal peristaltic bolus transport using a mix of experimental data, the conservation laws of mechanics and mathematical modeling. Whereas circular muscle tone generates radial closure pressure to create a local peristaltic closure wave, longitudinal muscle tone has two functions, one physiological with mechanical implications, and one purely mechanical. Each of these functions independently reduces the tension of individual circular muscle fibers to maintain closure as a consequence of shortening of longitudinal muscle locally coordinated with increasing circular muscle tone. The physiological function is deduced by combining basic laws of mechanics with concurrent measurements of intraluminal pressure from manometry, and changes in cross sectional muscle area from endoluminal ultrasound from which local longitudinal shortening (LLS) can be accurately obtained. The purely mechanical function of LLS was discovered from mathematical modeling of peristaltic esophageal transport with the axial wall motion generated by LLS. Physiologically, LLS concentrates circular muscle fibers where closure pressure is highest. However, the mechanical function of LLS is to reduce the level of pressure required to maintain closure. The combined physiological and mechanical consequences of LLS are to reduce circular muscle fiber tension and power by as much as 1/10 what would be required for peristalsis without the longitudinal muscle layer, a tremendous benefit that may explain the existence of longitudinal muscle fiber in the gut. We also review what is understood of the role of longitudinal muscle in esophageal emptying, reflux and pathology.
Figures
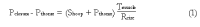
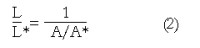
References
-
- Nicosia MA, Brasseur JG, Liu JB, Miller LS. Local longitudinal muscle shortening of the human esophagus from high-frequency ultrasonography. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1022–G1033. - PubMed
-
- Dai Q, Korimilli A, Thangada VK, Chung CY, Parkman H, Brasseur J, Miller LS. Muscle shortening along the normal esophagus during swallowing. Dig Dis Sci. 2006;51:105–109. - PubMed
-
- Ulerich R, Dai Q, Miller LS, Brasseur JG. Detailed 3-D Anatomy of the Human Gastro-Esophageal Segment. Gastroenterology. 2003;124:A259–A259.
-
- Liu JB, Miller LS, Goldberg BB, Feld RI, Alexander AA, Needleman L, Castell DO, Klenn PJ, Millward CL. Transnasal US of the esophagus: preliminary morphologic and function studies. Radiology. 1992;184:721–727. - PubMed
-
- Miller LS, Liu JB, Colizzo FP, Ter H, Marzano J, Barbarevech C, Helwig K, Leung L, Goldberg BB, Hedwig K corrected to Helwig K. Correlation of high-frequency esophageal ultrasonography and manometry in the study of esophageal motility. Gastroenterology. 1995;109:832–837. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

