Mononuclear Cu-O2 complexes: geometries, spectroscopic properties, electronic structures, and reactivity
- PMID: 17458929
- PMCID: PMC2593863
- DOI: 10.1021/ar700008c
Mononuclear Cu-O2 complexes: geometries, spectroscopic properties, electronic structures, and reactivity
Abstract
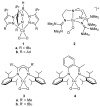
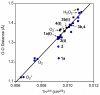
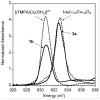
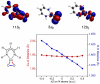
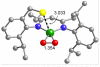
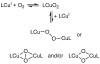
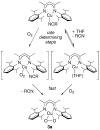
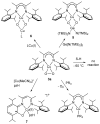
Using interwoven experimental and theoretical methods, detailed studies of several structurally defined 1:1 Cu-O 2 complexes have provided important fundamental chemical information useful for understanding the nature of intermediates involved in aerobic oxidations in synthetic and enzymatic copper-mediated catalysis. In particular, these studies have shed new light on the factors that influence the mode of O 2 coordination (end-on vs side-on) and the electronic structure, which can vary between Cu(II)-superoxo and Cu(III)-peroxo extremes.
Figures

References
-
-
(a) Selected reviews:Isabel B, Carrondo MA, Peter FL. Reduction of dioxygen by enzymes containing copper. J. Biol. Inorg. Chem. 2006;11:539–547.Whittaker JW. Free Radical Catalysis by Galactose Oxidase. Chem. Rev. 2003;103:2347–2363.Solomon EI, Chen P, Metz M, Lee S-K, Palmer AE. Oxygen binding, activation, and reduction to water by copper proteins. Angew. Chem. Int. Ed. 2001;40:4570–4590.Halcrow M, Phillips S, Knowles P. Amine oxidases and galactose oxidase. In: Holzenburg A, Scrutton NS, editors. Subcellular Biochemistry, Vol. 35: Enzyme-Catalyzed Electron and Radical Transfer. Plenum; New York: 2000. pp. 183–231.Fontecave M, Pierre JL. Oxidations by Copper Metalloenzymes and some Biomimetic Approaches. Coord. Chem. Rev. 1998;170:125–140.Solomon EI, Sundaram UM, Machonkin TE. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996;96:2563–2605.Klinman JP. Mechanisms whereby mononuclear copper proteins functionalize organic substrates. Chem. Rev. 1996;96:2541–2561.
-
-
-
(a) Illustrative examples:Punniyamurthy T, Velusamy S, Iqbal J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005;105:2329–2364.and references cited therein.Markó IE, Gautier A, Dumeunier R, Doda K, Philippart F, Brown SM, Urch CJ. Efficient, Copper-Catalyzed, Aerobic Oxidation of Primary Alcohols. Angew. Chem. Int. Ed. 2004;43:1588–1591.Murahashi S-I, Komiya N, Hayashi Y, Kumano T. Copper complexes for catalytic, aerobic oxidation of hydrocarbons. Pure Appl. Chem. 2001;73:311–314.Gupta R, Mukherjee R. Catalytic Oxidation of Hindered Phenols by a Copper(I) Complex and Dioxygen. Tetrahedron Lett. 2000;41:7763–7767.
-
-
- Mirica LM, Ottenwaelder X, Stack TDP. Structure and Spectroscopy of Copper-Dioxygen Complexes. Chem. Rev. 2004;104:1013–1045. - PubMed
- Lewis EA, Tolman WB. Reactivity of Copper-Dioxygen Systems. Chem. Rev. 2004;104:1047–1076. - PubMed
- Hatcher L, Karlin KD. Oxidant types in copper-dioxygen chemistry: the ligand coordination defines the Cun-O2 structure and subsequent reactivity. J. Biol. Inorg. Chem. 2004;9:669–683. - PubMed
- Osako T, Terada S, Tosha T, Nagatomo S, Furutachi H, Fujinami S, Kitagawa T, Suzukib M, Itoh S. Structure and dioxygen-reactivity of copper(I) complexes supported by bis(6-methylpyridin-2-ylmethyl)amine tridentate ligands. Dalton Trans. 2005:3514–3521. - PubMed
- Itoh S, Fukuzumi S. Dioxygen activation by copper complexes. Mechanistic insights into copper monooxygenases and copper oxidases. Bull. Chem. Soc. Jpn. 2002;75:2081–2095.
- Schindler S. Reactivity of Copper(I) Complexes Towards Dioxygen. Eur. J. Inorg. Chem. 2000:2311–2326.
- Que L, Jr., Tolman WB. Bis(μ-oxo)dimetal “diamond” cores in copper and iron complexes relevant to biocatalysis. Angew. Chem. Int. Ed. 2002;41:1114–1137. - PubMed
-
- Karlin KD, Tolman WB, Kaderli S, Zuberbühler AD. Kinetic and thermodynamic parameters of copper-dioxygen interaction with different oxygen binding modes. J. Mol. Catal. A. 1997;117:215–222.
-
- Itoh S. Mononuclear copper active-oxygen complexes. Curr. Opin. Chem. Biol. 2006;10:115–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

