CXC chemokine ligand 16 in periodontal diseases: expression in diseased tissues and production by cytokine-stimulated human gingival fibroblasts
- PMID: 17459077
- PMCID: PMC1942022
- DOI: 10.1111/j.1365-2249.2007.03398.x
CXC chemokine ligand 16 in periodontal diseases: expression in diseased tissues and production by cytokine-stimulated human gingival fibroblasts
Abstract
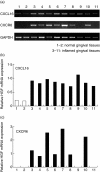
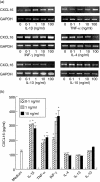
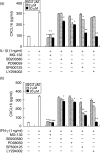
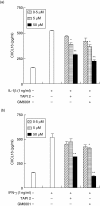
Periodontal disease is an inflammatory disorder characterized by the involvement of chemokines that are important for the recruitment of leucocytes. Several cytokines are involved in regulating levels of chemokines in periodontal disease. CXCL16 is a chemokine related to the migration of T helper 1 (Th1) cells and natural killer (NK) cells. In this study, we examined its expression in periodontal tissues. Moreover, we investigated the effects of cytokines on the production of CXCL16 by human gingival fibroblast (HGF). Reverse transcription-polymerase chain reaction (RT-PCR) analysis and immunohistochemistry revealed that CXCL16 and its receptor, CXCR6, were expressed at the mRNA and protein levels in diseased tissues. Proinflammatory cytokines [interleukin (IL)-1beta, tumour necrosis factor (TNF)-alpha and interferon (IFN)-gamma] increased the mRNA expression and release of CXCL16 in a dose-dependent manner. Moreover, treatment of HGFs with IFN-gamma in combination with IL-1beta had a synergistic effect on the production of CXCL16. On the other hand, IL-4 and IL-13 inhibited the IL-1beta-induced CXCL16 production by HGFs. Inhibitors of A disintegrin and metalloprotease (ADAM)10 and ADAM17, a recently identified protease of CXCL16, reduced the amount of CXCL16 released from HGFs. These results suggest that the CXCL16 produced by HGFs may be involved in the migration of leucocytes into inflamed tissues, and provide evidence that CXCL16 production is controlled by cytokines in periodontal disease.
Figures


References
-
- Dzink JL, Tanner AC, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985;12:648–59. - PubMed
-
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. - PubMed
-
- Kjeldsen M, Holmstrup P, Bendtzen K. Marginal periodontitis and cytokines: a review of the literature. J Periodontol. 1993;64:1013–22. - PubMed
-
- Wilson M, Reddi K, Henderson B. Cytokine-inducing components of periodontopathogenic bacteria. J Periodont Res. 1996;31:393–407. - PubMed
-
- Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

