Distinct phospholipase A2 enzymes regulate prostaglandin E2 and F2alpha production by bovine endometrial epithelial cells
- PMID: 17459165
- PMCID: PMC1868772
- DOI: 10.1186/1477-7827-5-16
Distinct phospholipase A2 enzymes regulate prostaglandin E2 and F2alpha production by bovine endometrial epithelial cells
Abstract
Background: The rate-limiting step in prostaglandin (PG) biosynthesis is catalyzed by phospholipase A2 (PLA2) enzymes which hydrolyze arachidonic acid from membrane phospholipids. Despite their importance in uterine PG production, little is known concerning the specific PLA2 enzymes that regulate arachidonic acid liberation in the uterine endometrium. The objectives of this study were to evaluate the expression and activities of calcium-independent Group VI and Group IVC PLA2 (PLA2G6 and PLA2G4C) and calcium-dependent Group IVA PLA2 (PLA2G4A) enzymes in the regulation of bovine uterine endometrial epithelial cell PG production.
Methods: Bovine endometrial epithelial cells in culture were treated with oxytocin, interferon-tau and the PLA2G6 inhibitor bromoenol lactone, alone and in combination. Concentrations of PGF2alpha and PGE2 released into the medium were analyzed. Western blot analysis was performed on cellular protein to determine the effects of treatments on expression of PLA2G4A, PLA2G6 and PLA2G4C. Group-specific PLA2 activity assays were performed on cell lysates following treatment with oxytocin, interferon-tau or vehicle (control), alone and in combination. To further evaluate the role of specific PLA2 enzymes in uterine cell PG biosynthesis, cells were transfected with cDNAs encoding human PLA2G6 and PLA24C, treated as described above and PG assays performed.
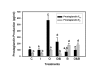
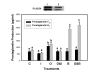
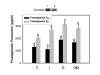
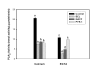
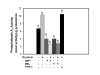
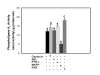
Results: Constitutive cell production of PGF2alpha was about two-fold higher than PGE2. Oxytocin stimulated production of both PGs but the increase of PGF2alpha was significantly greater. Interferon-tau diminished oxytocin stimulation of both PGs. The PLA2G6 inhibitor, bromoenol lactone, abolished oxytocin-stimulated production of PGF2alpha. Treatments had little effect on PLA2G4A protein expression. In contrast, oxytocin enhanced expression of PLA2G6 and this effect was diminished in the presence of interferon-tau. Expression of PLA2G4C was barely detectable in control and oxytocin treated cells but it was enhanced in cells treated with interferon-tau. Oxytocin stimulated PLA2 activity in assays designed to evaluate PLA2G6 activity and interferon-tau inhibited this response. In assays designed to measure PLA2G4C activity, only interferon-tau was stimulatory. Cells overexpressing PLA2G6 produced similar quantities of the two PGs and these values were significantly higher than PG production by non-transfected cells. Oxytocin stimulated production of both PGs and this response was inhibited by interferon-tau. Bromoenol lactone inhibited oxtocin stimulation of PGF2alpha production but stimulated PGE2 production, both in the absence and presence of oxytocin. Cells over-expressing PLA2G4C produced more PGE2 than PGF2alpha and interferon-tau stimulated PGE2 production.
Conclusion: Results from these studies indicate that oxytocin stimulation of uterine PGF2alpha production is mediated, at least in part, by up-regulation of PLA2G6 expression and activity. In addition to its known inhibitory effect on oxytocin receptor expression, interferon-tau represses oxytocin-stimulated PLA2G6 expression and activity and this contributes to diminished PGF2alpha production. Furthermore, endometrial cell PGE2 biosynthesis was associated with PLA2G4C expression and activity and interferon-tau was stimulatory to this process.
Figures

Similar articles
-
Phospholipase A2 regulation of bovine endometrial (BEND) cell prostaglandin production.Reprod Biol Endocrinol. 2008 Sep 23;6:44. doi: 10.1186/1477-7827-6-44. Reprod Biol Endocrinol. 2008. PMID: 18811942 Free PMC article.
-
Recombinant ovine and bovine interferons tau regulate prostaglandin production and oxytocin response in cultured bovine endometrial cells.Biol Reprod. 1997 Feb;56(2):402-8. doi: 10.1095/biolreprod56.2.402. Biol Reprod. 1997. PMID: 9116139
-
In vitro response to oxytocin and interferon-Tau in bovine endometrial cells from caruncular and inter-caruncular areas.Biol Reprod. 1998 Aug;59(2):241-7. doi: 10.1095/biolreprod59.2.241. Biol Reprod. 1998. PMID: 9687291
-
Uterine-conceptus interactions and reproductive failure in cattle.Theriogenology. 2001 Dec 1;56(9):1435-50. doi: 10.1016/s0093-691x(01)00645-8. Theriogenology. 2001. PMID: 11768809 Review.
-
Maternal recognition of pregnancy.J Reprod Fertil Suppl. 1995;49:15-28. J Reprod Fertil Suppl. 1995. PMID: 7623310 Review.
Cited by
-
Prostaglandins regulate humoral immune responses in Aedes aegypti.PLoS Negl Trop Dis. 2020 Oct 23;14(10):e0008706. doi: 10.1371/journal.pntd.0008706. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33095767 Free PMC article.
-
Primate-specific evolution of noncoding element insertion into PLA2G4C and human preterm birth.BMC Med Genomics. 2010 Dec 24;3:62. doi: 10.1186/1755-8794-3-62. BMC Med Genomics. 2010. PMID: 21184677 Free PMC article.
-
Dynamic regulation of the transcriptome and proteome of the equine embryo during maternal recognition of pregnancy.FASEB Bioadv. 2022 Oct 18;4(12):775-797. doi: 10.1096/fba.2022-00063. eCollection 2022 Dec. FASEB Bioadv. 2022. PMID: 36479207 Free PMC article.
-
EP3 (E-Prostanoid 3) Receptor Mediates Impaired Vasodilation in a Mouse Model of Salt-Sensitive Hypertension.Hypertension. 2021 Apr;77(4):1399-1411. doi: 10.1161/HYPERTENSIONAHA.120.16518. Epub 2021 Mar 1. Hypertension. 2021. PMID: 33641369 Free PMC article.
-
Lipid-Based Molecules on Signaling Pathways in Autism Spectrum Disorder.Int J Mol Sci. 2022 Aug 29;23(17):9803. doi: 10.3390/ijms23179803. Int J Mol Sci. 2022. PMID: 36077195 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

