Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy
- PMID: 17459355
- PMCID: PMC2778048
- DOI: 10.1016/j.cardiores.2007.03.019
Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy
Abstract
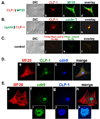
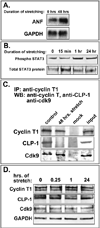
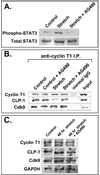
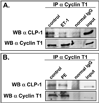
Objective: Our aim was to determine if the expression pattern of CLP-1 in developing heart is consistent with its role in controlling RNA transcript elongation by transcriptional elongation factor b (P-TEFb) and if the inhibitory control exerted over P-TEFb by CLP-1 is released under hypertrophic conditions.
Methods: We performed immunoblot and immunofluorescence analysis of CLP-1 and the P-TEFb components cdk9 and cyclin T in fetal mouse heart and 2 day post-natal mouse cardiomyocytes to determine if they are co-localized. We induced hypertrophy in rat cardiomyocytes either by mechanical stretch or treatment with hypertrophic agents such as endothelin-1 and phenylephrine to determine if CLP-1 is released from P-TEFb in response to hypertrophic stimuli. The involvement of the Jak/STAT signal transduction pathway in this process was studied by blocking this pathway with the Jak2 kinase inhibitor, AG490, and assessing the association of CLP-1 with P-TEFb complexes.
Results: We found that CLP-1 is expressed along with P-TEFb components in developing heart during the period in which knockout mice lacking the CLP-1 gene develop cardiac hypertrophy and die. Under conditions of hypertrophy induced by mechanical stretch or agonist treatment, CLP-1 dissociates from the P-TEFb complex, a finding consistent with the de-repression of P-TEFb kinase activity seen in hypertrophic cardiomyocytes. Blockage of Jak/STAT signaling by AG490 prevented release of CLP-1 from P-TEFb despite the ongoing presence of hypertrophic stimulation by mechanical stretch.
Conclusions: CLP-1 expression in developing heart and isolated post-natal cardiomyocytes colocalizes with P-TEFb expression and therefore has the potential to regulate RNA transcript elongation by controlling P-TEFb cdk9 kinase activity in heart. We further conclude that the dissociation of CLP-1 from P-TEFb is responsive to hypertrophic stimuli transduced by cellular signal transduction pathways. This process may be part of the genomic stress response resulting in increased RNA transcript synthesis in hypertrophic cardiomyocytes.
Figures


Similar articles
-
P-TEFb: The master regulator of transcription elongation.Mol Cell. 2023 Feb 2;83(3):393-403. doi: 10.1016/j.molcel.2022.12.006. Epub 2023 Jan 3. Mol Cell. 2023. PMID: 36599353 Free PMC article. Review.
-
Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1.Circ Res. 2009 Jun 19;104(12):1347-54. doi: 10.1161/CIRCRESAHA.108.191726. Epub 2009 May 14. Circ Res. 2009. PMID: 19443839 Free PMC article.
-
Differential Activation of P-TEFb Complexes in the Development of Cardiomyocyte Hypertrophy following Activation of Distinct G Protein-Coupled Receptors.Mol Cell Biol. 2020 Jun 29;40(14):e00048-20. doi: 10.1128/MCB.00048-20. Print 2020 Jun 29. Mol Cell Biol. 2020. PMID: 32341082 Free PMC article.
-
Cyclins that don't cycle--cyclin T/cyclin-dependent kinase-9 determines cardiac muscle cell size.Cell Cycle. 2003 Mar-Apr;2(2):99-104. Cell Cycle. 2003. PMID: 12695656 Review.
-
The positive transcription elongation factor b is an essential cofactor for the activation of transcription by myocyte enhancer factor 2.J Mol Biol. 2008 Oct 3;382(2):275-87. doi: 10.1016/j.jmb.2008.07.017. Epub 2008 Jul 16. J Mol Biol. 2008. PMID: 18662700 Free PMC article.
Cited by
-
P-TEFb: The master regulator of transcription elongation.Mol Cell. 2023 Feb 2;83(3):393-403. doi: 10.1016/j.molcel.2022.12.006. Epub 2023 Jan 3. Mol Cell. 2023. PMID: 36599353 Free PMC article. Review.
-
BET acetyl-lysine binding proteins control pathological cardiac hypertrophy.J Mol Cell Cardiol. 2013 Oct;63:175-9. doi: 10.1016/j.yjmcc.2013.07.017. Epub 2013 Aug 9. J Mol Cell Cardiol. 2013. PMID: 23939492 Free PMC article.
-
The JAK-STAT pathway in hypertrophic stress signaling and genomic stress response.JAKSTAT. 2012 Apr 1;1(2):131-41. doi: 10.4161/jkst.20702. JAKSTAT. 2012. PMID: 24058762 Free PMC article. Review.
-
Down-regulation of cardiac lineage protein (CLP-1) expression in CLP-1 +/- mice affords.J Cell Mol Med. 2009 Aug;13(8B):2744-2753. doi: 10.1111/j.1582-4934.2008.00404.x. J Cell Mol Med. 2009. PMID: 18624753 Free PMC article.
-
CDK9 keeps RNA polymerase II on track.Cell Mol Life Sci. 2021 Jul;78(14):5543-5567. doi: 10.1007/s00018-021-03878-8. Epub 2021 Jun 19. Cell Mol Life Sci. 2021. PMID: 34146121 Free PMC article. Review.
References
-
- Schaub MC, Hefti MA, Zuellig RA, Morano I. Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc Res. 1998;37:381–404. - PubMed
-
- Lompre A, Mercadier J, Schwartz K. Changes in Gene Expression during Cardiac Growth. Int Rev Cyto. 1991;124:137–185. - PubMed
-
- Molkentin JD, Dorn II GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. - PubMed
-
- Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

