Mast cell degranulation activates a pain pathway underlying migraine headache
- PMID: 17459586
- PMCID: PMC2045157
- DOI: 10.1016/j.pain.2007.03.012
Mast cell degranulation activates a pain pathway underlying migraine headache
Abstract
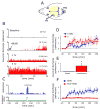
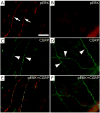
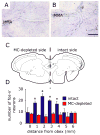
Intracranial headaches such as that of migraine are generally accepted to be mediated by prolonged activation of meningeal nociceptors but the mechanisms responsible for such nociceptor activation are poorly understood. In this study, we examined the hypothesis that meningeal nociceptors can be activated locally through a neuroimmune interaction with resident mast cells, granulated immune cells that densely populate the dura mater. Using in vivo electrophysiological single unit recording of meningeal nociceptors in the rat we observed that degranulation of dural mast cells using intraperitoneal administration of the basic secretagogue agent compound 48/80 (2 mg/kg) induced a prolonged state of excitation in meningeal nociceptors. Such activation was accompanied by increased expression of the phosphorylated form of the extracellular signal-regulated kinase (pERK), an anatomical marker for nociceptor activation. Mast cell-induced nociceptor interaction was also associated with downstream activation of the spinal trigeminal nucleus as indicated by an increase in c-fos expression. Our findings provide evidence linking dural mast cell degranulation to prolonged activation of the trigeminal pain pathway believed to underlie intracranial headaches such as that of migraine.
Figures



References
-
- Artico M, Cavallotti C. Catecholaminergic and acetylcholine esterase containing nerves of cranial and spinal dura mater in humans and rodents. Microsc Res Tech. 2001;53:212–20. - PubMed
-
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. - PubMed
-
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–42. - PubMed
-
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

