Sulfite oxidizing enzymes
- PMID: 17459792
- PMCID: PMC1993547
- DOI: 10.1016/j.bbapap.2007.03.006
Sulfite oxidizing enzymes
Abstract
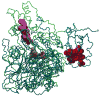
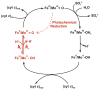
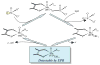
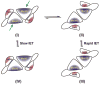
Sulfite oxidizing enzymes are essential mononuclear molybdenum (Mo) proteins involved in sulfur metabolism of animals, plants and bacteria. There are three such enzymes presently known: (1) sulfite oxidase (SO) in animals, (2) SO in plants, and (3) sulfite dehydrogenase (SDH) in bacteria. X-ray crystal structures of enzymes from all three sources (chicken SO, Arabidopsis thaliana SO, and Starkeya novella SDH) show nearly identical square pyramidal coordination around the Mo atom, even though the overall structures of the proteins and the presence of additional cofactors vary. This structural information provides a molecular basis for studying the role of specific amino acids in catalysis. Animal SO catalyzes the final step in the degradation of sulfur-containing amino acids and is critical in detoxifying excess sulfite. Human SO deficiency is a fatal genetic disorder that leads to early death, and impaired SO activity is implicated in sulfite neurotoxicity. Animal SO and bacterial SDH contain both Mo and heme domains, whereas plant SO only has the Mo domain. Intraprotein electron transfer (IET) between the Mo and Fe centers in animal SO and bacterial SDH is a key step in the catalysis, which can be studied by laser flash photolysis in the presence of deazariboflavin. IET studies on animal SO and bacterial SDH clearly demonstrate the similarities and differences between these two types of sulfite oxidizing enzymes. Conformational change is involved in the IET of animal SO, in which electrostatic interactions may play a major role in guiding the docking of the heme domain to the Mo domain prior to electron transfer. In contrast, IET measurements for SDH demonstrate that IET occurs directly through the protein medium, which is distinctly different from that in animal SO. Point mutations in human SO can result in significantly impaired IET or no IET, thus rationalizing their fatal effects. The recent developments in our understanding of sulfite oxidizing enzyme mechanisms that are driven by a combination of molecular biology, rapid kinetics, pulsed electron paramagnetic resonance (EPR), and computational techniques are the subject of this review.
Figures
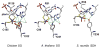

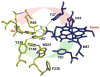
References
-
- Hille R. The mononuclear molybdenum enzymes. Chem Rev. 1996;96:2757–2816. - PubMed
-
- Aguey-Zinsou KF, Bernhardt PV, Kappler U, McEwan AG. Direct electrochemistry of a bacterial sulfite dehydrogenase. J Am Chem Soc. 2003;125:530–535. - PubMed
-
- Eilers T, Schwarz G, Brinkmann H, Witt C, Richter T, Nieder J, Koch B, Hille R, Hansch R, Mendel RR. Identification and biochemical characterization of Arabidopsis thaliana sulfite oxidase - A new player in plant sulfur metabolism. J Biol Chem. 2001;276:46989–46994. - PubMed
-
- Hansch R, Lang C, Riebeseel E, Lindigkeit R, Gessler A, Rennenberg H, Mendel RR. Plant sulfite oxidase as novel producer of H2O2 - Combination of enzyme catalysis with a subsequent non-enzymatic reaction step. J Biol Chem. 2006;281:6884–6888. - PubMed
-
- Kisker C. Sulfite oxidase. In: Messerschmidt A, Huber R, Poulos T, Wieghardt K, editors. Handbook of Metalloproteins. John Wiley and Sons, Ltd; New York: 2001. pp. 1121–1135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

