Chemical synthesis of a very long oligoribonucleotide with 2-cyanoethoxymethyl (CEM) as the 2'-O-protecting group: structural identification and biological activity of a synthetic 110mer precursor-microRNA candidate
- PMID: 17459888
- PMCID: PMC1904286
- DOI: 10.1093/nar/gkm202
Chemical synthesis of a very long oligoribonucleotide with 2-cyanoethoxymethyl (CEM) as the 2'-O-protecting group: structural identification and biological activity of a synthetic 110mer precursor-microRNA candidate
Abstract
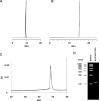
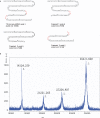
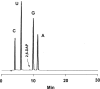
A long RNA oligomer, a 110mer with the sequence of a precursor-microRNA candidate, has been chemically synthesized in a single synthesizer run by means of standard automated phosphoramidite chemistry. The synthetic method involved the use of 2-cyanoethoxymethyl (CEM), a 2'-hydroxyl protecting group recently developed in our laboratory. We improved the methodology, introducing better coupling and capping conditions. The overall isolated yield of highly pure 110mer was 5.5%. Such a yield on a 1-mumol scale corresponds to 1 mg of product and emphasizes the practicality of the CEM method for synthesizing oligomers of more than 100 nt in sufficient quantity for biological research. We confirmed the identity of the 110mer by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, as well as HPLC, electrophoretic methods, and RNase-digestion experiments. The 110mer also showed sense-selective specific gene-silencing activity. As far as we know, this is the longest chemically synthesized RNA oligomer reported to date. Furthermore, the identity of the 110mer was confirmed by both physicochemical and biological methods.
Figures



Similar articles
-
Chemical synthesis of a very long RNA oligomer, a 110mer precursor-miRNA candidate, with 2-cyanoethoxymethyl (CEM) as the 2'-O-protecting group.Nucleic Acids Symp Ser (Oxf). 2007;(51):3-4. doi: 10.1093/nass/nrm002. Nucleic Acids Symp Ser (Oxf). 2007. PMID: 18029557
-
A novel RNA synthetic method with a 2'-O-(2-cyanoethoxymethyl) protecting group.Nucleic Acids Symp Ser (Oxf). 2006;(50):11-2. doi: 10.1093/nass/nrl006. Nucleic Acids Symp Ser (Oxf). 2006. PMID: 17150792
-
Chemical synthesis of oligoribonucleotides with 2'-O-(2-cyanoethoxymethyl)-protected phosphoramidites.Curr Protoc Nucleic Acid Chem. 2008 Sep;Chapter 2:Unit 2.15. doi: 10.1002/0471142700.nc0215s34. Curr Protoc Nucleic Acid Chem. 2008. PMID: 18819083
-
Preparation of 5'-silyl-2'-orthoester ribonucleosides for use in oligoribonucleotide synthesis.Curr Protoc Nucleic Acid Chem. 2004 May;Chapter 2:Unit 2.10. doi: 10.1002/0471142700.nc0210s16. Curr Protoc Nucleic Acid Chem. 2004. PMID: 18428924 Review.
-
Synthesis of 8-oxoguanosine phosphoramidite and its incorporation into Oligoribonucleotides.Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:4.58.1-10. doi: 10.1002/0471142700.nc0458s56. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25606978 Review.
Cited by
-
HPLC methods for purity evaluation of man-made single-stranded RNAs.Sci Rep. 2019 Jan 31;9(1):1019. doi: 10.1038/s41598-018-37642-z. Sci Rep. 2019. PMID: 30705318 Free PMC article.
-
Solid-Phase-Supported Chemoenzymatic Synthesis of a Light-Activatable tRNA Derivative.Angew Chem Int Ed Engl. 2022 Jan 3;61(1):e202111613. doi: 10.1002/anie.202111613. Epub 2021 Nov 22. Angew Chem Int Ed Engl. 2022. PMID: 34738704 Free PMC article.
-
Combined Approaches to Site-Specific Modification of RNA.ACS Chem Biol. 2008 Jan 18;3(1):30-37. doi: 10.1021/cb7002225. Epub 2008 Jan 5. ACS Chem Biol. 2008. PMID: 18177002 Free PMC article.
-
An Unconventional Acid-Labile Nucleobase Protection Concept for Guanosine Phosphoramidites in RNA Solid-Phase Synthesis.Chemistry. 2017 Mar 8;23(14):3406-3413. doi: 10.1002/chem.201605056. Epub 2017 Feb 9. Chemistry. 2017. PMID: 27943429 Free PMC article.
-
Strategies for Covalent Labeling of Long RNAs.Chembiochem. 2021 Oct 1;22(19):2826-2847. doi: 10.1002/cbic.202100161. Epub 2021 Jun 17. Chembiochem. 2021. PMID: 34043861 Free PMC article. Review.
References
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA Interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- Hammond SM. MicroRNA therapeutics: a new niche for antisense nucleic acids. Trends Mol. Med. 2006;12:99–101. - PubMed
-
- The FANTOM Consortium and RIKEN Genome Exploration Research Group and Genome Science Group. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. - PubMed
-
- RIKEN Genome Exploration Research Group and Genome Science Group and the FANTOM Consortium. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

