Transient synaptic silencing of developing striate cortex has persistent effects on visual function and plasticity
- PMID: 17460066
- PMCID: PMC6672996
- DOI: 10.1523/JNEUROSCI.0772-07.2007
Transient synaptic silencing of developing striate cortex has persistent effects on visual function and plasticity
Abstract
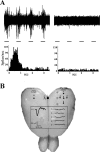
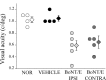
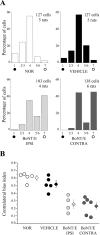
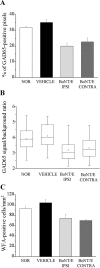
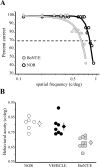
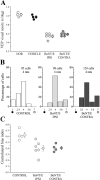
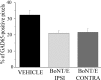
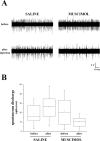
Neural circuits in the cerebral cortex are shaped by experience during "critical periods" early in life. For example, visual cortex is immature at the time of eye opening and gradually develops its functional properties during a sensitive period. Very few reports have addressed the role of intrinsic neural activity in cortical maturation. Here we have exploited the bacterial enzyme botulinum neurotoxin E (BoNT/E) to produce a unilateral, reversible blockade of neural activity in rat visual cortex during the sensitive period. BoNT/E is a highly selective protease that interferes with transmitter release via cleavage of the synaptic protein SNAP-25 (synaptosomal-associated protein of 25 kDa). Unilateral, intracortical injections of BoNT/E were made at the time of eye opening and resulted in the silencing of the treated, but not contralateral, hemisphere for a period of 2 weeks. We found that visual acuity was permanently reduced in the blocked hemisphere, and the critical period for ocular dominance plasticity persisted into adulthood. Unexpectedly, these effects extended equally to the contralateral, uninjected side, demonstrating a fundamental role for interhemispheric connections in cortical maturation.
Figures




References
-
- Benevento LA, Bakkum BW, Port JD, Cohen RS. The effects of dark-rearing on the electrophysiology of the rat visual corte. Brain Re. 1992;572:198–207. - PubMed
-
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. - PubMed
-
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. - PubMed
-
- Berlucchi G, Rizzolatti G. Binocularly driven neurons in visual cortex of split-chiasm cats. Science. 1968;159:308–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
