Spinal interneurons that are selectively activated during fictive flexion reflex
- PMID: 17460076
- PMCID: PMC6673003
- DOI: 10.1523/JNEUROSCI.5602-06.2007
Spinal interneurons that are selectively activated during fictive flexion reflex
Abstract
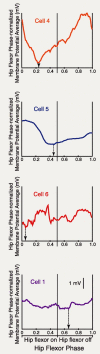
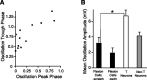
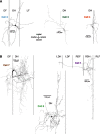
Behavioral choices in invertebrates are mediated by a combination of shared and specialized circuitry, including neurons that are inhibited during competing behaviors. Less is known, however, about the neural mechanisms of behavioral choice in vertebrates. The spinal cord can appropriately select among several types of limb movements, including limb withdrawal (flexion reflex), scratching, and locomotion, and thus is conducive to examination of vertebrate mechanisms of behavioral choice. Flexion reflex can interrupt and reset the rhythm of scratching and locomotion, suggesting that a combination of shared and specialized circuitry contributes to these behaviors, but little is known about the interneurons involved. Here, I used in vivo intracellular recording and dye injection to identify a group of spinal interneurons that are strongly activated during fictive flexion reflex but inhibited during fictive scratching and fictive swimming. These flexion-selective interneurons are typically rhythmically hyperpolarized during fictive scratching and fictive swimming. This hyperpolarization can be maximal during the ipsilateral hip flexor bursts of rhythmic limb motor patterns, although these cells are strongly activated during the ipsilateral hip flexor bursts of fictive flexion reflex. Thus, these interneurons are relatively specialized for fictive limb withdrawal, rather than contributing to the hip flexor phase of multiple types of limb movements. These flexion-selective cells are physiologically and morphologically distinguishable from a recently described group of spinal interneurons (transverse interneurons) that are strongly activated during both fictive flexion reflex and fictive scratching. Thus, spinal interneurons with distinct behavioral roles may to some extent be morphologically distinguishable.
Figures




References
-
- Berkowitz A. Broadly tuned spinal neurons for each form of fictive scratching in spinal turtles. J Neurophysiol. 2001;86:1017–1025. - PubMed
-
- Berkowitz A. Both shared and specialized spinal circuitry for scratching and swimming in turtles. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:225–234. - PubMed
-
- Berkowitz A. Physiology and morphology indicate that individual spinal interneurons contribute to diverse limb movements. J Neurophysiol. 2005;94:4455–4470. - PubMed
-
- Berkowitz A. Physiology and morphology of spinal interneurons that are preferentially activated during fictive flexion reflex. Soc Neurosci Abstr. 2006;32:448–26.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
