BK channels with beta3a subunits generate use-dependent slow afterhyperpolarizing currents by an inactivation-coupled mechanism
- PMID: 17460083
- PMCID: PMC6672991
- DOI: 10.1523/JNEUROSCI.0758-07.2007
BK channels with beta3a subunits generate use-dependent slow afterhyperpolarizing currents by an inactivation-coupled mechanism
Abstract
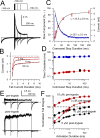
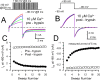
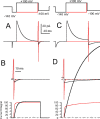
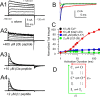
Large-conductance, Ca2+- and voltage-activated K+ (BK) channels are broadly expressed proteins that respond to both cellular depolarization and elevations in cytosolic Ca2+. The characteristic functional properties of BK channels among different cells are determined, in part, by tissue-specific expression of auxiliary beta subunits. One important functional property conferred on BK channels by beta subunits is inactivation. Yet, the physiological role of BK channel inactivation remains poorly understood. Here we report that as a consequence of a specific mechanism of inactivation, BK channels containing the beta3a auxiliary subunit exhibit an anomalous slowing of channel closing. This produces a net repolarizing current flux that markedly exceeds that expected if all open channels had simply closed. Because of the time dependence of inactivation, this behavior results in a Ca2+-independent but time-dependent increase in a slow tail current, providing an unexpected mechanism by which use-dependent changes in slow afterhyperpolarizations might regulate electrical firing. The physiological significance of inactivation in BK channels mediated by different beta subunits may therefore arise not from inactivation itself, but from the differences in the amplitude and duration of repolarizing currents arising from the beta-subunit-specific energetics of recovery from inactivation.
Figures




Similar articles
-
Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels.J Neurosci. 2002 Mar 1;22(5):1550-61. doi: 10.1523/JNEUROSCI.22-05-01550.2002. J Neurosci. 2002. PMID: 11880485 Free PMC article.
-
Modulation of BK Channel Function by Auxiliary Beta and Gamma Subunits.Int Rev Neurobiol. 2016;128:51-90. doi: 10.1016/bs.irn.2016.03.015. Epub 2016 Apr 8. Int Rev Neurobiol. 2016. PMID: 27238261 Free PMC article. Review.
-
Differential effects of beta 1 and beta 2 subunits on BK channel activity.J Gen Physiol. 2005 Apr;125(4):395-411. doi: 10.1085/jgp.200409236. Epub 2005 Mar 14. J Gen Physiol. 2005. PMID: 15767297 Free PMC article.
-
Molecular mechanism underlying β1 regulation in voltage- and calcium-activated potassium (BK) channels.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4809-14. doi: 10.1073/pnas.1504378112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825713 Free PMC article.
-
Calcium-activated potassium channels in adrenal chromaffin cells.Ion Channels. 1996;4:261-301. doi: 10.1007/978-1-4899-1775-1_7. Ion Channels. 1996. PMID: 8744211 Review.
Cited by
-
Interactions between beta subunits of the KCNMB family and Slo3: beta4 selectively modulates Slo3 expression and function.PLoS One. 2009 Jul 3;4(7):e6135. doi: 10.1371/journal.pone.0006135. PLoS One. 2009. PMID: 19578543 Free PMC article.
-
Regulation of BK Channels by Beta and Gamma Subunits.Annu Rev Physiol. 2019 Feb 10;81:113-137. doi: 10.1146/annurev-physiol-022516-034038. Annu Rev Physiol. 2019. PMID: 30742788 Free PMC article. Review.
-
Stereospecific binding of a disordered peptide segment mediates BK channel inactivation.Nature. 2012 May 3;485(7396):133-6. doi: 10.1038/nature10994. Nature. 2012. PMID: 22522931 Free PMC article.
-
Species-specific Differences among KCNMB3 BK beta3 auxiliary subunits: some beta3 N-terminal variants may be primate-specific subunits.J Gen Physiol. 2008 Jul;132(1):115-29. doi: 10.1085/jgp.200809969. J Gen Physiol. 2008. PMID: 18591419 Free PMC article.
-
Regulatory γ1 subunits defy symmetry in functional modulation of BK channels.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):9923-9928. doi: 10.1073/pnas.1804560115. Epub 2018 Sep 17. Proc Natl Acad Sci U S A. 2018. PMID: 30224470 Free PMC article.
References
-
- Demo SD, Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991;7:743–753. - PubMed
-
- Faraldo-Gomez JD, Kutluay E, Jogini V, Zhao Y, Heginbotham L, Roux B. Mechanism of intracellular block of the KcsA K+ channel by tetrabutylammonium: insights from X-ray crystallography, electrophysiology and replica-exchange molecular dynamics simulations. J Mol Biol. 2007;365:649–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
