Ambient light regulates sodium channel activity to dynamically control retinal signaling
- PMID: 17460088
- PMCID: PMC3232015
- DOI: 10.1523/JNEUROSCI.0183-07.2007
Ambient light regulates sodium channel activity to dynamically control retinal signaling
Abstract
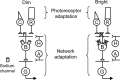
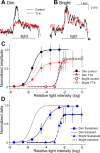
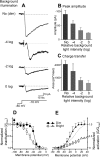
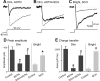
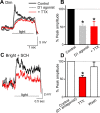
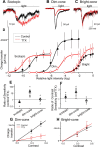
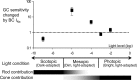
The retinal network increases its sensitivity in low-light conditions to detect small visual inputs and decreases its sensitivity in bright-light conditions to prevent saturation. However, the cellular mechanisms that adjust visual signaling in the retinal network are not known. Here, we show that voltage-gated sodium channels in bipolar cells dynamically control retinal light sensitivity. In dim conditions, sodium channels amplified light-evoked synaptic responses mediated by cone pathways. Conversely, in bright conditions, sodium channels were inactivated by dopamine released from amacrine cells, and they did not amplify synaptic inputs, minimizing signal saturation. Our findings demonstrate that bipolar cell sodium channels mediate light adaptation by controlling retinal signaling gain.
Figures
Similar articles
-
Dopamine D1 receptor activation contributes to light-adapted changes in retinal inhibition to rod bipolar cells.J Neurophysiol. 2018 Aug 1;120(2):867-879. doi: 10.1152/jn.00855.2017. Epub 2018 May 30. J Neurophysiol. 2018. PMID: 29847232 Free PMC article.
-
Inhibitory components of retinal bipolar cell receptive fields are differentially modulated by dopamine D1 receptors.Vis Neurosci. 2020 Feb 12;37:E01. doi: 10.1017/S0952523819000129. Vis Neurosci. 2020. PMID: 32046810 Free PMC article.
-
Dopamine D1 receptor activation reduces local inner retinal inhibition to light-adapted levels.J Neurophysiol. 2019 Apr 1;121(4):1232-1243. doi: 10.1152/jn.00448.2018. Epub 2019 Feb 6. J Neurophysiol. 2019. PMID: 30726156 Free PMC article.
-
Voltage-gated Na channels in AII amacrine cells accelerate scotopic light responses mediated by the rod bipolar cell pathway.J Neurosci. 2010 Mar 31;30(13):4650-9. doi: 10.1523/JNEUROSCI.4212-09.2010. J Neurosci. 2010. PMID: 20357115 Free PMC article.
-
D1 dopamine receptors modulate cone ON bipolar cell Nav channels to control daily rhythms in photopic vision.Chronobiol Int. 2015 Feb;32(1):48-58. doi: 10.3109/07420528.2014.951054. Epub 2014 Aug 26. Chronobiol Int. 2015. PMID: 25157610
Cited by
-
Dopamine D1 receptor expression is bipolar cell type-specific in the mouse retina.J Comp Neurol. 2016 Jul 1;524(10):2059-79. doi: 10.1002/cne.23932. Epub 2015 Dec 8. J Comp Neurol. 2016. PMID: 26587737 Free PMC article.
-
Reduced Retinal Function in the Absence of Na(v)1.6.PLoS One. 2012;7(2):e31476. doi: 10.1371/journal.pone.0031476. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355369 Free PMC article.
-
M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina.J Neurosci. 2016 Jul 6;36(27):7184-97. doi: 10.1523/JNEUROSCI.3500-15.2016. J Neurosci. 2016. PMID: 27383593 Free PMC article.
-
Voltage- and calcium-gated ion channels of neurons in the vertebrate retina.Prog Retin Eye Res. 2019 Sep;72:100760. doi: 10.1016/j.preteyeres.2019.05.001. Epub 2019 May 10. Prog Retin Eye Res. 2019. PMID: 31078724 Free PMC article. Review.
-
NaV1.1 channels in axon initial segments of bipolar cells augment input to magnocellular visual pathways in the primate retina.J Neurosci. 2013 Oct 9;33(41):16045-59. doi: 10.1523/JNEUROSCI.1249-13.2013. J Neurosci. 2013. PMID: 24107939 Free PMC article.
References
-
- Baccus SA, Meister M. Fast and slow contrast adaptation in retinal circuitry. Neuron. 2002;36:909–919. - PubMed
-
- Boatright JH, Hoel MJ, Iuvone PM. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res. 1989;482:164–168. - PubMed
-
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. - PubMed
-
- Cantrell AR, Ma JY, Scheuer T, Catterall WA. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron. 1996;16:1019–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
