Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines
- PMID: 17460660
- PMCID: PMC2631661
- DOI: 10.1038/nature05813
Chemical glycosylation in the synthesis of glycoconjugate antitumour vaccines
Abstract
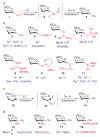
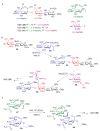
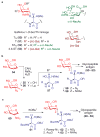
Therapeutic vaccines derived from carbohydrate antigen-adjuvant combinations are a promising approach for cancer immunotherapy. One of the critical limitations in this area is access to sufficient quantities of tumour-associated carbohydrate antigens and glycoconjugate adjuvants. At present, availability of the complex oligosaccharide constructs that are needed for the systematic design and evaluation of novel vaccine formulations relies on de novo chemical synthesis. The use of both state-of-the-art and emerging glycosylation technologies has led to significant advances in this field, allowing the clinical exploration of carbohydrate-based antigens in the treatment of cancer.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314:53–57. - PubMed
-
- Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside–KLH conjugate vaccines. Immunol Rev. 1995;145:147–166. - PubMed
-
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. - PubMed
-
- Fung PY, Madej M, Koganty RR, Longenecker BM. Active specific immunotherapy of a murine mammary adenocarcinoma using a synthetic tumor-associated glycoconjugate. Cancer Res. 1990;50:4308–4314. - PubMed
-
- Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83:418–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

