Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells
- PMID: 17460778
- PMCID: PMC1854847
- DOI: 10.1593/neo.06823
Oncogenic K-Ras signals through epidermal growth factor receptor and wild-type H-Ras to promote radiation survival in pancreatic and colorectal carcinoma cells
Abstract
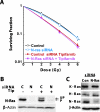
Pancreatic and colorectal carcinomas frequently express oncogenic/mutant K-Ras that contributes to both tumorigenesis and clinically observed resistance to radiation treatment. We have previously shown that farnesyltransferase inhibitors (FTI) radiosensitize many pancreatic and colorectal cancer cell lines that express oncogenic K-ras at doses that inhibit the prenylation and activation of H-Ras but not K-Ras. In the present study, we have examined the mechanism of FTI-mediated radiosensitization in cell lines that express oncogenic K-Ras and found that wild-type H-Ras is a contributor to radiation survival in tumor cells that express oncogenic K-Ras. In these experiments, inhibiting the expression of oncogenic K-Ras, wild-type H-Ras, or epidermal growth factor receptor (EGFR) led to similar levels of radiosensitization as treatment with the FTI tipifarnib. Treatment with the EGFR inhibitor gefitinib led to similar levels of radiosensitization, and the combinations of tipifarnib or gefitinib plus inhibition of K-Ras, H-Ras, or EGFR expression did not provide additional radiosensitization compared with tipifarnib or gefitinib alone. Finally, supplementing culture medium with the EGFR ligand transforming growth factor alpha was able to reverse the radiosensitizing effect of inhibiting K-ras expression. Taken together, these findings suggest that EGFR-activated H-Ras signaling is initiated by oncogenic K-Ras to promote radiation survival in pancreatic and colorectal cancers.
Figures



Similar articles
-
Pancreatic cancer cell radiation survival and prenyltransferase inhibition: the role of K-Ras.Cancer Res. 2005 Sep 15;65(18):8433-41. doi: 10.1158/0008-5472.CAN-05-0158. Cancer Res. 2005. PMID: 16166322
-
Farnesyltransferase inhibitor (L-744,832) restores TGF-beta type II receptor expression and enhances radiation sensitivity in K-ras mutant pancreatic cancer cell line MIA PaCa-2.Oncogene. 2002 Nov 7;21(51):7883-90. doi: 10.1038/sj.onc.1205948. Oncogene. 2002. PMID: 12420225
-
Radiosensitization of epidermal growth factor receptor/HER2-positive pancreatic cancer is mediated by inhibition of Akt independent of ras mutational status.Clin Cancer Res. 2010 Feb 1;16(3):912-23. doi: 10.1158/1078-0432.CCR-09-1324. Epub 2010 Jan 26. Clin Cancer Res. 2010. PMID: 20103665 Free PMC article.
-
Farnesyltransferase inhibitors as radiation sensitizers.Semin Radiat Oncol. 2002 Jul;12(3 Suppl 2):27-32. doi: 10.1053/srao.2002.34866. Semin Radiat Oncol. 2002. PMID: 12174342 Review.
-
[The RAS paradox of the EGFR-targeted therapy in colorectal cancer].Magy Onkol. 2008 Jun;52(2):185-91. doi: 10.1556/MOnkol.52.2008.2.7. Magy Onkol. 2008. PMID: 18640895 Review. Hungarian.
Cited by
-
Killing of Kras-mutant colon cancer cells via Rac-independent actin remodeling by the βGBP cytokine, a physiological PI3K inhibitor therapeutically effective in vivo.Mol Cancer Ther. 2012 Sep;11(9):1884-93. doi: 10.1158/1535-7163.MCT-11-1041-T. Epub 2012 Jul 2. Mol Cancer Ther. 2012. PMID: 22752425 Free PMC article.
-
The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth.Front Cell Dev Biol. 2021 Sep 27;9:743907. doi: 10.3389/fcell.2021.743907. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34646829 Free PMC article. Review.
-
Tumor vascular changes mediated by inhibition of oncogenic signaling.Cancer Res. 2009 Aug 1;69(15):6347-54. doi: 10.1158/0008-5472.CAN-09-0657. Epub 2009 Jul 21. Cancer Res. 2009. PMID: 19622766 Free PMC article.
-
Targeting K-Ras-mediated DNA damage response in radiation oncology: Current status, challenges and future perspectives.Clin Transl Radiat Oncol. 2022 Oct 17;38:6-14. doi: 10.1016/j.ctro.2022.10.004. eCollection 2023 Jan. Clin Transl Radiat Oncol. 2022. PMID: 36313934 Free PMC article. Review.
-
The War on Cancer rages on.Neoplasia. 2009 Dec;11(12):1252-63. doi: 10.1593/neo.91866. Neoplasia. 2009. PMID: 20019833 Free PMC article.
References
-
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. - PubMed
-
- Cengel KA, McKenna WG. Molecular targets for altering radiosensitivity: lessons from Ras as a pre-clinical and clinical model. Crit Rev Oncol Hematol. 2005;55:103–116. - PubMed
-
- Brunner TB, Hahn SM, Gupta AK, Muschel RJ, McKenna WG, Bernhard EJ. Farnesyltransferase inhibitors: an overview of the results of preclinical and clinical investigations. Cancer Res. 2003;63:5656–5668. - PubMed
-
- Sebti SM, Der CJ. Opinion: searching for the elusive targets of farnesyltransferase inhibitors. Nat Rev Cancer. 2003;3:945–951. - PubMed
-
- Bernhard EJ, Kao G, Cox AD, Sebti SM, Hamilton AD, Muschel RJ, McKenna WG. The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras-transformed rat embryo fibroblasts. Cancer Research. 1996;56:1727–1730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
