Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells
- PMID: 17460779
- PMCID: PMC1854851
- DOI: 10.1593/neo.07133
Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells
Abstract
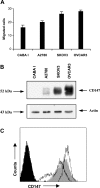
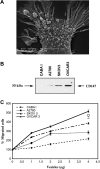
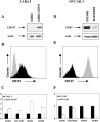
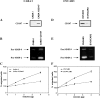
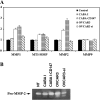
Matrix metalloproteinase (MMP) degradation of extracellular matrix is thought to play an important role in invasion, angiogenesis, tumor growth, and metastasis. Several studies have demonstrated that CD147/extracellular MMP inducer, a membrane-spanning molecule highly expressed in tumor cells, may be involved in the progression of malignancies by regulating expression of MMP in peritumoral stromal cells. In the present study we show that CD147 is expressed in microvesicles derived from epithelial ovarian cancer cells and that CD147-positive vesicles may promote an angiogenic phenotype in endothelial cells in vitro. Vesicles shed by human ovarian carcinoma cell lines OVCAR3, SKOV3, and A2780 expressed different levels of CD147 and stimulated proangiogenic activities of human umbilical vein endothelial cells (HUVECs) in a CD147-dependent fashion (OVCAR3 > SKOV3 > A2780). Moreover, vesicles shed by ovarian carcinoma cell line CABA I with low CD147 expression had no significant effect on the development of angiogenic phenotype in HUVECs. The treatment of OVCAR3 cells with small interfering RNA against CD147 suppressed the angiogenic potential of OVCAR3-derived microvesicles. However, transfection of CD147 cDNA into the CABA I cell line enabled CABA I-derived vesicles to induce angiogenesis and to promote MMP genes expression in HUVECs. We therefore conclude that vesicles shed by ovarian cancer cells may induce proangiogenic activities of HUVECs by a CD147-mediated mechanism.
Figures

References
-
- Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. - PubMed
-
- Toole BP. Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr Top Dev Biol. 2003;54:371–389. - PubMed
-
- Davidson B, Goldberg I, Berner A, Kristensen GB, Reich R. EMMPRIN (extracellular matrix metalloproteinase inducer) is a novel marker of poor outcome in serous ovarian carcinoma. Clin Exp Metastasis. 2003;20:161–169. - PubMed
-
- Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004;2:73–80. - PubMed
-
- Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–2281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
