Black cohosh and St. John's wort (GYNO-Plus) for climacteric symptoms
- PMID: 17461529
- PMCID: PMC2628120
- DOI: 10.3349/ymj.2007.48.2.289
Black cohosh and St. John's wort (GYNO-Plus) for climacteric symptoms
Abstract
Purpose: This study was conducted to investigate the efficacy of black cohosh (Cimicifuga racemosa) and St. John's wort (Hypericum perforatum) in women with climacteric symptoms, and to assess their effects on vaginal atrophy, hormone levels, and lipid profiles.
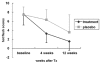
Materials and methods: In this double-blind randomized, placebo-controlled, multicenter study, 89 peri- or postmenopausal women experiencing climacteric symptoms were treated with St. John's wort and black cohosh extract (Gynoplus), Jin-Yang Pharm., Seoul, Korea) or a matched placebo for 12 weeks. Climacteric complaints were evaluated by the Kupperman Index (KI) initially and at 4 and 12 weeks following treatment. Vaginal maturation indices, serum estradiol, FSH, LH, total cholesterol, HDL- cholesterol, LDL-cholesterol, and triglyceride levels were measured before and after treatment. From the initial 89 participants, 77 completed the trial (42 in the Gynoplus group, 35 in the placebo group).
Results: Baseline characteristics were not significantly different between the two groups. Mean KI scores and hot flushes after 4 and 12 weeks were significantly lower in the Gynoplus group. Differences in superficial cell proportion were not statistically significant. HDL levels decreased in the control group from 60.20 +/- 16.37 to 56.63 +/- 12.67, and increased in the Gynoplus group from 58.32 +/- 11.64 to 59.74 +/- 10.54; this was statistically significant (p=0.04).
Conclusion: Black cohosh and St. John's wort combination was found to be effective in alleviating climacteric symptoms and might provide benefits to lipid metabolism.
Figures
References
-
- Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29:319–336. - PubMed
-
- Cauley JA, Norton L, Lippman ME, Eckert S, Krueger KA, Purdie DW, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001;65:125–134. - PubMed
-
- Herrington DM, Klein KP. Cardiovascular trials of estrogen replacement therapy. Ann N Y Acad Sci. 2001;949:153–162. - PubMed
-
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. - PubMed
-
- Liehr JG. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001;7:273–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical