Lacosamide: a review of preclinical properties
- PMID: 17461888
- PMCID: PMC6494128
- DOI: 10.1111/j.1527-3458.2007.00001.x
Lacosamide: a review of preclinical properties
Abstract
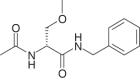
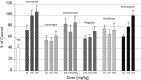
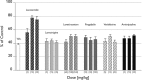
Lacosamide (LCM), (SPM 927, (R)-2-acetamido-N-benzyl-3-methoxypropionamide, previously referred to as harkoseride or ADD 234037) is a member of a series of functionalized amino acids that were specifically synthesized as anticonvulsive drug candidates. LCM has demonstrated antiepileptic effectiveness in different rodent seizure models and antinociceptive potential in experimental animal models that reflect distinct types and symptoms of neuropathic as well as chronic inflammatory pain. Recent results suggest that LCM has a dual mode of action underlying its anticonvulsant and analgesic activity. It was found that LCM selectively enhances slow inactivation of voltage-gated sodium channels without affecting fast inactivation. Furthermore, employing proteomic affinity-labeling techniques, collapsin-response mediator protein 2 (CRMP-2 alias DRP-2) was identified as a binding partner. Follow-up experiments confirmed a functional interaction of LCM with CRMP-2 in vitro. LCM did not inhibit or induce a wide variety of cytochrome P450 enzymes at therapeutic concentrations. In safety pharmacology and toxicology studies conducted in mice, rats, rabbits, and dogs, LCM was well tolerated. Either none or only minor side effects were observed in safety studies involving the central nervous, respiratory, gastrointestinal, and renal systems and there is no indication of abuse liability. Repeated dose toxicity studies demonstrated that after either intravenous or oral administration of LCM the adverse events were reversible and consisted mostly of exaggerated pharmacodynamic effects on the CNS. No genotoxic or carcinogenic effects were observed in vivo, and LCM showed a favorable profile in reproductive and developmental animal studies. Currently, LCM is in a late stage of clinical development as an adjunctive treatment for patients with uncontrolled partial-onset seizures, and it is being assessed as monotherapy in patients with painful diabetic neuropathy. Further trials to identify LCM's potential in pain and for other indications have been initiated.
Figures

References
-
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E (1998) Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA 280:1831–1836. - PubMed
-
- Barton ME, Klein BD, Wolf HH, White HS (2001) Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 47:217–227. - PubMed
-
- Bausch SB (2005) Axonal sprouting of GABAergic interneurons in temporal lobe epilepsy. Epilepsy Behav 7:390–400. - PubMed
-
- Ben‐Menachem E, Biton V, Jatuzis D, Abou‐Khalil B, Doty P, Rudd GD (2005) Efficacy and safety of adjunctive oral lacosamide for the treatment of partial onset seizures in patients with epilepsy. Epilepsia 57:30. - PubMed
-
- Beyreuther B, Callizot N, Stohr T (2006) Antinociceptive efficacy of lacosamide in a rat model for painful diabetic neuropathy. Eur J Pharmacol 539:64–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

