Spin-labeled analogs of CMP-NeuAc as NMR probes of the alpha-2,6-sialyltransferase ST6Gal I
- PMID: 17462576
- PMCID: PMC3968682
- DOI: 10.1016/j.chembiol.2007.02.010
Spin-labeled analogs of CMP-NeuAc as NMR probes of the alpha-2,6-sialyltransferase ST6Gal I
Abstract
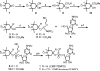
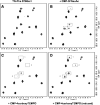
Structural data on mammalian proteins are often difficult to obtain by conventional NMR approaches because of an inability to produce samples with uniform isotope labeling in bacterial expression hosts. Proteins with sparse isotope labels can be produced in eukaryotic hosts by using isotope-labeled forms of specific amino acids, but structural analysis then requires information from experiments other than nuclear Overhauser effects. One source of alternate structural information is distance-dependent perturbation of spin relaxation times by nitroxide spin-labeled analogs of natural protein ligands. Here, we introduce spin-labeled analogs of sugar nucleotide donors for sialyltransferases, specifically, CMP-TEMPO (CMP-4-O-[2,2,6,6-tetramethylpiperidine-1-oxyl]) and CMP-4carboxyTEMPO (CMP-4-O-[4-carboxy-2,2,6,6-tetramethylpiperidinine-1-oxyl]). An ability to identify resonances from active site residues and produce distance constraints is illustrated on a (15)N phenylalanine-labeled version of the structurally uncharacterized, alpha-2,6-linked sialyltransferase, ST6Gal I.
Figures
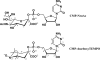
References
-
- Moseley HNB, Monleon D, Montelione GT. Automatic determination of protein backbone resonance assignments from triple resonance nuclear magnetic resonance data. Methods Enzymol. 2001;339:91–108. - PubMed
-
- Wider G. NMR techniques used with very large biological macromolecules in solution. Methods Enzymol. 2005;394:382–398. - PubMed
-
- Lustbader JW, Birken S, Pollak S, Pound A, Chait BT, Mirza UA, Ramnarain S, Canfield RE, Brown JM. Expression of human chorionic gonadotropin uniformly labeled with NMR isotopes in Chinese hamster ovary cells: an advance toward rapid determination of glycoprotein structures. J. Biomol. NMR. 1996;7:295–304. - PubMed
-
- Mao HY, Gunasekera AH, Fesik SW. Expression, refolding, and isotopic labeling of human serum albumin domains for NMR spectroscopy. Protein Expr. Purif. 2000;20:492–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

