GATA6 is an astrocytoma tumor suppressor gene identified by gene trapping of mouse glioma model
- PMID: 17463088
- PMCID: PMC1876570
- DOI: 10.1073/pnas.0611669104
GATA6 is an astrocytoma tumor suppressor gene identified by gene trapping of mouse glioma model
Abstract
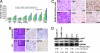
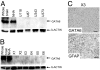
Malignant astrocytomas are the most common and lethal adult primary brain tumor. Retroviral gene trapping of nontransformed neonatal astrocytes from a glial fibrillary acidic protein (GFAP):(V12)Ha-Ras murine astrocytoma model led to isolation of the transcription factor Gata6. Loss of Gata6 resulted in enhanced proliferation and transformation of astrocytes. Human malignant astrocytoma cell lines, explant xenografts, and operative specimens demonstrated loss of GATA6 expression. Loss-of-function GATA6 mutations with loss of heterozygosity of the GATA6 locus were found in human malignant astrocytoma specimens but not in lower-grade astrocytomas or normal adult astrocytes. Knockdown of Gata6 expression in (V12)Ha-Ras or p53-/- astrocytes, but not in parental murine or human astrocytes, led to acceleration of tumorgenesis. Knockin GATA6 expression in human malignant astrocytoma cells reduced their tumorgenic growth with decreased VEGF expression. Collectively, these data demonstrate that GATA6, isolated from a murine astrocytoma model, is a novel tumor suppressor gene that is a direct target of mutations during malignant progression of murine and human astrocytomas. This work also demonstrates the utility of random mutagenesis strategies, such as gene trapping, on murine cancer models toward discovery of novel genetic alterations in corresponding human cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Trapping the mouse genome to hunt human alterations.Proc Natl Acad Sci U S A. 2007 May 8;104(19):7737-8. doi: 10.1073/pnas.0702617104. Epub 2007 May 3. Proc Natl Acad Sci U S A. 2007. PMID: 17483476 Free PMC article. No abstract available.
Similar articles
-
Trapping the mouse genome to hunt human alterations.Proc Natl Acad Sci U S A. 2007 May 8;104(19):7737-8. doi: 10.1073/pnas.0702617104. Epub 2007 May 3. Proc Natl Acad Sci U S A. 2007. PMID: 17483476 Free PMC article. No abstract available.
-
Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas.Cancer Res. 2001 May 1;61(9):3826-36. Cancer Res. 2001. PMID: 11325859
-
Pathological and molecular progression of astrocytomas in a GFAP:12 V-Ha-Ras mouse astrocytoma model.Am J Pathol. 2005 Sep;167(3):859-67. doi: 10.1016/S0002-9440(10)62057-3. Am J Pathol. 2005. PMID: 16127163 Free PMC article.
-
Roles of the functional loss of p53 and other genes in astrocytoma tumorigenesis and progression.Neuro Oncol. 1999 Apr;1(2):124-37. doi: 10.1093/neuonc/1.2.124. Neuro Oncol. 1999. PMID: 11550308 Free PMC article. Review.
-
Genetic alterations associated with glioma progression.Verh Dtsch Ges Pathol. 1994;78:43-7. Verh Dtsch Ges Pathol. 1994. PMID: 7534015 Review.
Cited by
-
Phosphorylation of the Hippo Pathway Component AMOTL2 by the mTORC2 Kinase Promotes YAP Signaling, Resulting in Enhanced Glioblastoma Growth and Invasiveness.J Biol Chem. 2015 Aug 7;290(32):19387-401. doi: 10.1074/jbc.M115.656587. Epub 2015 May 21. J Biol Chem. 2015. PMID: 25998128 Free PMC article.
-
Dimorphic glioblastoma with glial and epithelioid phenotypes: Clonal evolution and immune selection.Front Neurol. 2023 Jan 10;13:1017087. doi: 10.3389/fneur.2022.1017087. eCollection 2022. Front Neurol. 2023. PMID: 36703629 Free PMC article.
-
Loss of GATA6 leads to nuclear deformation and aneuploidy in ovarian cancer.Mol Cell Biol. 2009 Sep;29(17):4766-77. doi: 10.1128/MCB.00087-09. Epub 2009 Jul 6. Mol Cell Biol. 2009. PMID: 19581290 Free PMC article.
-
Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours.Br J Cancer. 2009 Aug 18;101(4):722-33. doi: 10.1038/sj.bjc.6605179. Epub 2009 Jul 14. Br J Cancer. 2009. PMID: 19603027 Free PMC article.
-
Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients.J Clin Invest. 2012 Jan;122(1):253-66. doi: 10.1172/JCI59334. Epub 2011 Dec 12. J Clin Invest. 2012. PMID: 22156195 Free PMC article.
References
-
- Mahaley MS, Jr, Mettlin C, Natarajan N, Laws ER, Jr, Peace BB. J Neurosurg. 1989;71:826–836. - PubMed
-
- Kleihues P, Cavenee WK, editors. WHO Classification of Tumors: Pathology and Genetics of Tumours of the Nervous System. Lyon, France: Int Agency for Res on Cancer; 2000.
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. N Engl J Med. 2005;352:987–996. - PubMed
-
- Louis DN, Gusella JF. Trends Genet. 1995;11:412–415. - PubMed
-
- Guha A, Feldkamp MM, Lau N, Boss G, Pawson A. Oncogene. 1997;15:2755–2765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

