Seven-transmembrane receptors and ubiquitination
- PMID: 17463329
- PMCID: PMC1952537
- DOI: 10.1161/01.RES.0000261939.88744.5a
Seven-transmembrane receptors and ubiquitination
Abstract
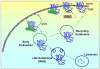
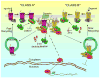
Regulation of protein function by posttranslational modification plays an important role in many biological pathways. The most well known among such modifications is protein phosphorylation performed by highly specific protein kinases. In the past decade, however, covalent linkage of the low-molecular-weight protein ubiquitin to substrate proteins (protein ubiquitination) has proven to be yet another widely used mechanism of protein regulation playing a crucial role in virtually all aspects of cellular functions. This review highlights some of the recently discovered and provocative roles for ubiquitination in the regulation of the life cycle and signal transduction properties of 7-transmembrane receptors that serve to integrate many biological functions and play fundamental roles in cardiovascular homeostasis.
Figures




Similar articles
-
Minireview: ubiquitination-regulated G protein-coupled receptor signaling and trafficking.Mol Endocrinol. 2013 Apr;27(4):558-72. doi: 10.1210/me.2012-1404. Epub 2013 Mar 7. Mol Endocrinol. 2013. PMID: 23471539 Free PMC article. Review.
-
G-Protein Dependent Signal Transduction and Ubiquitination in Dictyostelium.Int J Mol Sci. 2017 Oct 19;18(10):2180. doi: 10.3390/ijms18102180. Int J Mol Sci. 2017. PMID: 29048338 Free PMC article. Review.
-
Ubiquitination of G protein-coupled receptors: functional implications and drug discovery.Mol Pharmacol. 2012 Oct;82(4):563-70. doi: 10.1124/mol.112.079418. Epub 2012 Jun 14. Mol Pharmacol. 2012. PMID: 22700696 Free PMC article. Review.
-
The emerging complexity of protein ubiquitination.Biochem Soc Trans. 2009 Oct;37(Pt 5):937-53. doi: 10.1042/BST0370937. Biochem Soc Trans. 2009. PMID: 19754430 Review.
-
Regulation of G Protein-Coupled Receptors by Ubiquitination.Int J Mol Sci. 2017 Apr 27;18(5):923. doi: 10.3390/ijms18050923. Int J Mol Sci. 2017. PMID: 28448471 Free PMC article. Review.
Cited by
-
Role of α2-Adrenoceptor Subtypes in Suppression of L-Type Ca2+ Current in Mouse Cardiac Myocytes.Int J Mol Sci. 2021 Apr 16;22(8):4135. doi: 10.3390/ijms22084135. Int J Mol Sci. 2021. PMID: 33923625 Free PMC article.
-
The Role of E3, E4 Ubiquitin Ligase (UBE4B) in Human Pathologies.Cancers (Basel). 2019 Dec 24;12(1):62. doi: 10.3390/cancers12010062. Cancers (Basel). 2019. PMID: 31878315 Free PMC article. Review.
-
A novel EST-derived RNAi screen reveals a critical role for farnesyl diphosphate synthase in β2-adrenergic receptor internalization and down-regulation.FASEB J. 2012 May;26(5):1995-2007. doi: 10.1096/fj.11-193870. Epub 2012 Jan 25. FASEB J. 2012. PMID: 22278941 Free PMC article.
-
Brain-specific angiogenesis inhibitor-1 signaling, regulation, and enrichment in the postsynaptic density.J Biol Chem. 2013 Aug 2;288(31):22248-56. doi: 10.1074/jbc.M113.489757. Epub 2013 Jun 19. J Biol Chem. 2013. PMID: 23782696 Free PMC article.
-
MARCH2 promotes endocytosis and lysosomal sorting of carvedilol-bound β(2)-adrenergic receptors.J Cell Biol. 2012 Nov 26;199(5):817-30. doi: 10.1083/jcb.201208192. Epub 2012 Nov 19. J Cell Biol. 2012. PMID: 23166351 Free PMC article.
References
-
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. - PubMed
-
- Wise A, Gearing K, Rees S. Target validation of G-protein coupled receptors. Drug Discov Today. 2002;7:235–246. - PubMed
-
- Tang CM, Insel PA. GPCR expression in the heart; “new” receptors in myocytes and fibroblasts. Trends Cardiovasc Med. 2004;14:94–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

